Rare thrombophilic conditions
Introduction
Venous thromboembolism (VTE), which conventionally encompasses the two distinctive but frequently associated clinical entities deep vein thrombosis (DVT) and pulmonary embolism (PE), is characterized by the generation of a blood clot within the venous system. VTE is a serious worldwide healthcare issue, whose incidence is particularly high in hospitalized patients (1). Current statistics attests that VTE is ranked third among the leading cause of morbidity and mortality in Western countries, though the accurate estimation of its frequency is challenged by the often subtle and silent nature, which is then associated with a large burden of both misdiagnosis and underdiagnosis. Recent data suggests that its incidence is approximately ~1 per 1,000 adults annually, though a marked heterogeneity has been reported among different ethnic groups, as well as across ages, with an estimated frequency of 5–6 per 1,000 annually in subjects aged 80 years or older (2).
The term thrombophilia is conventionally used for describing a propensity for developing thrombosis due to the presence of either inherited or acquired hemostatic abnormalities, which may ultimately predispose to developing a transitory or permanent prothrombotic state (3). The identification of a condition of hereditary thrombophilia not necessarily implies that patients will certainly develop VTE at some point throughout their lifespan, since the different thrombophilic abnormalities are associated with considerably different risks of first lifetime or recurrent VTE (4). The accurate planning of type and duration of anticoagulant therapy necessitates a thoughtful comprehension of VTE pathogenesis, along with the possible identification of underlying thrombophilic risk factors. This conclusion is supported by data showing that approximately 50% of VTE have one or more hereditary or acquired thrombophilic conditions (5). Like many other human diseases, it has now been generally accepted that VTE pathogenesis is in essence multifactorial. Albeit a single risk factor may predispose to thrombosis, it is usually insufficient to trigger thrombosis, at least when present alone (2). The most studied congenital thrombophilic abnormalities can be typically classified in two ample classes: (I) those characterized by partial or complete loss of function, encompassing mutations causing abnormalities of endogenous anticoagulant proteins [i.e., antithrombin (AT), protein C (PC), and protein S (PS)]; and (II) gain-of-function mutations in clotting proteins, which mostly include factor V Leiden (FVL) and the prothrombin polymorphism G20210A (6,7).
Although many efforts have been made for studying many inherited and acquired risk factor over the past 30 years, increased commitment to investigating less frequent conditions has been encouraged by legislation aimed at facilitating patient care with more effective treatments, but has been paralleled by lack of initiatives from biotechnology and pharmaceutical companies for developing new drugs medicines that would not grant the same economic profit as for treating more frequent conditions (8).
Therefore, this review is aimed to discuss “less-frequent” thrombophilic risk factors, and presenting their epidemiologic, clinical and diagnostic characteristics.
Fibrinogen disorders (dysfibrinogenemia)
Fibrinogen, which is alternatively known as coagulation factor I, is a large (i.e., 340 kDa) and complex molecule formed by two identical subunits, linked by a disulfide bond, which plays an essential role in blood coagulation (secondary hemostasis) (9). This protein not only functions as fibrin precursor, thus stabilizing the blood clots, but also promotes platelet aggregation and fibrinolysis (10). As a whole, the conditions defined as dysfibrinogenemias encompass abnormalities of fibrinogen structure, which may variably result in an abnormal protein function. As for other thrombophilic conditions, these abnormalities can be both inherited or acquired. The latter conditions are frequently observed in patients with a kaleidoscope of underlying disorders, but is most commonly found as a consequence of liver diseases (11). The inherited fibrinogen abnormalities are usually rare, and may impair the concentration (hypofibrinogenemia), the activity (dysfibrinogenemia) of fibrinogen, or both (hypodysfibrinogenemia). Inherited fibrinogen disorder is usually classified as type I (afibrinogenemia and hypofibrinogenemia), which are characterized by an abnormal concentration of the protein, or as type II (dysfibrinogenemia and hypodysfibrinogenemia), which are instead associated with an abnormal function of the protein. A limited number of patients may have both dysfibrinogenemia and hypofibrinogenemia, a condition known as hypodysfibrinogenemia (12). The frequency of dysfibrinogenemia is typically low (i.e., approximately 8 per 1,000 individuals), so that routine dysfibrinogenemia testing in thrombophilic patients is not warranted (10). Although the first molecular basis of dysfibrinogenemia could be identified nearly 50 years ago, in 1968 and thus even before genetic sequence of the fibrinogen genes was initiated, the precise molecular basis of afibrinogenemia could only be unveil at a much later time (11).
Dysfibrinogenemia can be usually revealed by discrepancy between clotting and immunochemical fibrinogen tests. Nevertheless, this diagnosis is challenging even in highly specialized laboratories, because test sensitivity is dependent on the underlying mutation, as well as on reagents and techniques. The exact mechanism by which the risk for thrombosis is enhanced in patients with dysfibrinogenemia remains mostly unidentified, and perhaps depends on the nature of fibrinogen abnormalities (13). However, two leading mechanisms have been suggested for explaining the development of thrombosis in patients with abnormal fibrinogen, thus entailing that abnormal fibrinogen may have defective binding with thrombin, thus yielding to increased values of the second protein, and that the fibrin clot formed with “abnormal” fibrinogen may be less vulnerable to plasmin during tissue-type plasminogen activator (t-PA)-mediated fibrinolysis.
Heparin cofactor II (HCII)
HCII is a single chain glycoprotein, with an approximate size of 66.5 kDa, which belongs to a superfamily of serine protease inhibitors (i.e., serpins). Firstly identified in 1974 by Briginshaw and Shanberge, HCII is mainly produced by the hepatocytes, is present in the circulation at a concentration of approximately 1.0 µmol/L, has an estimated half-life ranging between 2–3 days, and functions as a rapid thrombin inhibitor in combination with heparin or dermatan sulfate (14,15). Unlike AT, which inhibits many proteases in blood coagulation cascade [i.e., especially factor (F) X and thrombin], HCII only acts as powerful thrombin inhibitor, whilst it lacks significant inhibitory function on other proteins of the coagulation cascade (16).
Despite many efforts made by many scientists, a straightforward association between HCII deficiency and venous thrombosis has not been defined so far (17), so that it may be reasonable to suggest that the role of HCII deficiency in venous thrombosis may be rather limited in vivo, unless combined with abnormalities of AT or associated with other inherited thrombotic risk factors.
An history of venous thromboembolic diseases has been identified in some subjects with partial inherited deficiency of HCII (i.e., approximately 50% of normal). Nevertheless, heterozygous HCII deficiency seems to be equally prevalent in both subjects with or without VTE, thus suggesting that HCII deficiency may not be a significant risk factor for this condition (15). Several studies analyzed the prevalence of HCII deficiency in VTE patients (i.e., in those with DVT, retinal vein thrombosis, cerebral venous sinus thrombosis). The prevalence of HCII deficiency was found to be mostly overlapping between patients and controls (i.e., 1.1% versus 1.0%), thus confirming that HCII deficiency should not be regarded as a strong thrombophilic condition. In some studies, the prevalence of HCII deficiency was compared between VTE patients and controls. Whilst Lopaciuk et al. found a significantly higher prevalence of HCII deficiency in thrombotic patients (5.7% versus 0.9%) (18), Ehrenforth et al. could only show a prevalence of 0.4% of HCII deficiency in 285 pediatric patients with venous or arterial thrombosis (19).
Thrombomodulin (TM)
TM is an endothelial glycosylated type I protein, which does not display intrinsic enzymatic activity. The TM linked to the endothelial membrane forms a high-affinity complex with thrombin, thus inhibiting its interaction with fibrinogen (20). When bound to TM, thrombin loses pro-coagulant activity and gain the capability to activate PC, a clinically essential endogenous anticoagulant protein (21). TM also exists in soluble form in plasma, originated by enzymatic cleavage of intact precursor (22). Several studies suggest that plasma soluble TM concentration, originating after cleavage from endothelial cells by neutrophil enzymes, may be associated with some pathological conditions characterized by vascular damage, including inflammation, infections and sepsis (23). It has been clearly shown that both the anticoagulant and pro-fibrinolytic activities of the thrombin-TM-PC system may play a pivotal role in preventing thromboembolic disorders. However, the most convincing evidence that lower TM function may be associated with thrombosis is only derived from animal studies (22). Kumada et al. provided clear evidence on the antithrombotic activity of TM in mice, by demonstrating that it actively participates to defense against thrombosis in vivo (24). Gomi et al. also showed that TM is highly effective in inhibiting thrombin-induced thromboembolism in mice, thus suggesting that recombinant human TM may be regarded as a powerful antithrombotic agent for being used both in vitro and in vivo (25). Weiler-Guettler et al. reported that transgenic mice with mutations in the TM gene (THBD) developed a prothrombotic condition and displayed enhanced fibrin deposition in target organs, a phenomenon that is probably attributable to lower capacity to generate activated PC (26). In another study, Isermann et al. showed that THBD-deficient mice experiment fast mortality after birth due to consumption coagulopathy (27).
Some other clinical studies have identified genetic abnormalities in THBD, but have also highlighted that these mutations have a questionable role in developing both venous and arterial thrombosis (28). In particular, the real impact of these polymorphisms on both concentration and activity of TM remains largely unclear. Ahmad et al. investigated the role of THBD c.1418C>T polymorphism in VTE recurrence, and found that it was not significantly associated with risk of VTE recurrence. Heit et al. carried out a large screening of unrelated patients with idiopathic DVT, for identifying mutations within the THBC gene (29), concluding that individual mutations or haplotypes within the THBC gene should not be regarded as major risk factors for VTE. Similar results were published by Le Flem et al., who also concluded that THBC polymorphisms, especially in the proximal promoter, are very infrequent in patients with VTE (30). A possible explanation for these findings is that severe THBC abnormalities, especially in the homozygous state, may be incompatible with life. This would lead us to conclude that, although the current experimental and clinical evidence supports a biologically plausible role of THBC gene mutations in developing thromboembolic disorders, the real impact of particular polymorphisms on TM function remains largely unproven.
Lipoprotein(a) [Lp(a)]
Lp(a) is a cholesterol-rich lipoprotein particle, consisting of a low-density lipoprotein (LDL) domain (containing apolipoprotein B-100) covalently bound to the glycoprotein apolipoprotein(a) [apo(a)] (31). Although part of the biochemical and biological characteristics of Lp(a) may reproduce those of LDL, the adjunctive presence of apo(a) makes this lipoprotein particle mostly unique (32), apo(a) is biochemically related to plasminogen, a key enzyme in the fibrinolytic system, and hence contains multiple tandem repeats of an amino acid sequence closely resembling plasminogen kringle IV, a unique kringle V domain, along with a sequence resembling the plasminogen protease domain (33). Due to high structural homology with plasminogen, Lp(a) is capable to inhibiting fibrinolysis by means of competing with plasminogen for stabilized fibrin binding. Interestingly, apo(a) and plasminogen are both members of a large superfamily of kringle-containing proteins, which also includes FXII, prothrombin, t-PA and urokinase (34). Although the plasma concentration of Lp(a) is predominantly genetically regulated, a number of acquired disorders (e.g., liver or renal impairment, hormonal derangements) may have an impact on synthesis and metabolism of the lipoprotein. Notably, the precise biological function of Lp(a) remains still largely unknown. The evidence that: (I) Lp(a) efficiently delivers cholesterol to a number of peripheral cells and (II) apo(a) reactive material can be identified close to surface of fibrous cap in atherogenic plaques, in endothelial cells and in granulation tissues during wound healing, supports the hypothesis that Lp(a) may play a role in tissue repair, and may have hence represented a kind of evolutionary advantage for species capable to synthesize apo(a) (31). Many data published over the past decades reflect a potential dichotomy of Lp(a) function, since both pro-atherosclerotic (LDL-like) and pro-thrombotic (plasminogen-like) functions have been described (35).
A convincing association has been widely reported between Lp(a) and a kaleidoscope of vascular occlusive disorders. Albeit the contribution of Lp(a) to the pathogenesis of atherosclerotic disease appears now virtually clear, uncertainty remains for other thrombotic pathologies (36).
Importantly, enhanced plasma Lp(a) concentration has been consistently shown to be an independent risk factor for coronary heart disease (CHD) in some prospective studies, but not in others. These inconsistencies may be attributable to a still existent lack of standardization of immunoassays, as well as to the strong dependency of test results from heterogeneity of apo(a) isoforms. In an earlier article, Seman et al. showed that Lp(a) is an independent risk factor form CHD in men, exhibiting a relative risk exceeding 2, whilst the association was found to be almost inconclusive in women (37). Seed et al. reinforced these findings, by demonstrating that increased Lp(a) concentration independently predicts future CHD in middle-aged men, with an odds ratio (OR) comprised between 1.9–2.3 (38). Although many convincing epidemiological data were published in favor of a causal role of Lp(a) in the pathogenesis of arterial occlusive disorders, data in support of an association with VTE are more contradictory. In a meta-analysis published by Sofi et al., including a discrete number of previous studies, a statistically significant association was observed between increased Lp(a) concentration and VTE (OR, 1.87) (39). More recently, Helgadottir et al. studied the effects of genetic variants of the apo(a) gene (LPA) on vascular disorders characterized by different atherosclerotic and thrombotic features, highlighting the existence of an association between specific genetic variants and atherosclerotic burden, whilst no association could be observed with thrombotic phenotypes (40). More recently, Dentali et al. carried out a systematic review and meta-analysis of the scientific literature, including data from 14 case-control studies, totaling 2,824 patients with VTE and 11,187 healthy matched controls, and reported a significant association between Lp(a) values and VTE. Nevertheless, the risk attributable to increased Lp(a) values appeared overall modest (i.e., 19.8%), thus providing further support to the still debated role of Lp(a) as risk factor of VTE (35).
Sticky platelet syndrome (SPS)
The SPS, an autosomal dominant platelet disorder associated with both arterial and venous thrombosis, is typically characterized by platelet hyperaggregability in platelet-rich plasma after being challenged with many platelet activators [i.e., adenosine diphosphate (ADP) and epinephrine] (2). SPS is a prothrombotic thrombocytopathy with familial occurrence, whose existence is not widely acknowledged, so that it may often remain underdiagnosed. Albeit patients with SPS usually experiment both arterial and venous thromboses at various sites, including coronary and cerebral arteries (41), this condition may also be associated with thrombosis in atypical sites such as cerebral sinuses, retina, as well as with peripheral arterial or VTE (42). The leading manifestation of SPS include signs and symptoms of venous or arterial thrombosis, migraine, along with pregnancy complications (43). The existence of SPS was originally reported at the 9th International Conference of Stroke and Cerebral Circulation in Arizona, in 1983, and was defined as a distinctive thrombotic disease attributable to qualitative abnormalities of platelet function, which would finally lead to enhanced aggregation and thrombosis (44). Although many studies on SPS have been published so far, epidemiological data remains mostly limited and the real prevalence of this condition in the general population is still largely unknown. Likewise, the prevalence of SPS in VTE patients can hardly be ascertained because available studies with significant sample size only investigated its prevalence in selected subpopulations (e.g., in those with unexplained thrombosis) (42). Interestingly, SPS has also been reported as a specific thrombophilic state associated with migraine with aura, which is increasingly diagnosed in relatives of patients with this syndrome (45).
Plasminogen activator inhibitor 1 (PAI-1)
The PAI-1, a serine protease inhibitor belonging to the serpin superfamily, is a selective inhibitor of both t-PA and urokinase-type plasminogen activator (u-PA). PAI-1 also interplays with non-proteinase targets including vitronectin, heparin and endocytic receptors of LDL-receptor family (46). PAI-1 is now being regarded as a multifunctional protein, which not only acts as endogenous regulator of both fibrinolysis and cell migration, but plays a role in some acute and chronic disorders (47). PAI-1 is synthesized by different cell types, including endothelial cells, fibroblasts, macrophages, adipocytes and hepatocytes. PAI-1 can also be released by platelets, and this may provide further explanations to the role of platelets in stabilizing blood clots (48). PAI-1 exhibits a very short half-life in plasma (i.e., approximately 7 minutes in active form), whilst the half-life of the latent form in circulation approximates 32 hours. At physiologic concentration, PAI-1 acts as a regulator of fibrinolysis, wound healing, matrix remodeling, as well as of cellular differentiation, proliferation and migration. Importantly, plasma PAI-1 concentration may increase from 5- to 10-fold during inflammatory, metabolic, ischemic or vascular disorders. An increasing body of evidence has contributed to define PAI-1 as an important mediator of organ fibrosis, thus strengthening its role in the pathogenesis of atherosclerosis, as well as in that of both arterial and venous thrombosis (49). Some family studies have earlier addressed PAI-1heritability, on the background of evidence linking genetics with PAI-1 concentration in plasma (48). More specifically, the 4G allele of the guanine insertion/deletion polymorphism (4G/5G) located within PAI-1 gene promoter has been associated with enhanced PAI values in plasma. Albeit a growing body of evidence has been brought to define PAI-1 as a major mediator of organ fibrosis, information on the association between the 4G allele and VTE risk remains mostly elusive. In particular, Sundquist et al. failed to find a significant association between the 4G/5G polymorphism and the risk of VTE in a cohort of unselected patients. Additional evidence hence suggests that the impact of PAI-1 polymorphism in VTE may be dependent on the presence of FVL (50). More recently, Tsantes et al. published a meta-analysis aimed to evaluate the association between 4G/5G polymorphism and risk of VTE, concluding that this allele appeared to modestly increase the risk of VTE (i.e., by approximately 15%), especially in subjects bearing additional genetic thrombophilic disorders (51).
In summary, the available evidence suggests that 4G/5G polymorphism may be a determinant of plasma PAI-1 concentration, which may then result in impaired fibrinolysis especially in the microcirculation, and may hence lead to a marginally increased risk of thrombosis in association with stressful events such as inflammation or in the presence of life-threatening diseases (52).
Apolipoprotein E (apo E)
Apo E is a 299-amino acid amphipathic protein belonging to the family of exchangeable apolipoproteins. Its leading function is mediating lipid transfer between circulating lipoproteins and tissues, through binding to cell receptors, which makes it a crucial player in transporting cholesterol and in assuring cell membrane maintenance and repair (53). Apo E, which is a key component of high-density lipoprotein (HDL), chylomicrons and very low-density lipoproteins (VLDL), is mainly synthesized in the liver both as free protein and in association with lipoproteins. Once in the circulation, apo E readily binds to, and dissociates from, lipoprotein surfaces. Apo E helps transporting lipids and facilitates the clearance of dietary fats, especially triglycerides, from the blood (54). The APOE gene exists in three major allelic forms, E2, E3 and E4. The relative frequency in humans of the relative alleles ε2, ε3 and ε4 is approximately 7%, 78% and 14%, respectively (55). ApoE3 is usually considered to the parent form, and is associated with normal plasma cholesterol values, whilst the function of ApoE2 and ApoE4 is impaired, and their presence is hence often associated with hyperlipidemia (54). APOE gene polymorphisms seem to have a role in cardiovascular disease due diminished ability of binding to Apo E receptors, thus leading to increased cholesterol level. The ApoE E2 allele (Cys-112 and Cys-158) exhibits a significantly lower binding ability; subjects bearing the E2/E2 combination thus remove dietary fats at a slower rate and are seemingly at an enhanced risk of developing early vascular disorders and type III hyperlipoproteinemia (56). The APOE gene polymorphisms have been associated with many other disorders. Some meta-analyses have recently been published, whose results suggest the existence of an association between APOE gene polymorphisms and hypertension (57), cerebral infarction (58), frontotemporal lobar degeneration (59), and vascular dementia (60). Regarding VTE, Zhu et al. studied 300 patients with DVT and 300 age- and gender-matched healthy controls, concluding that the APOE E3/E4 genotype was associated with an enhanced risk for DVT (OR, 1.48) (55). Similar evidence was published by Nagato et al. in a small case-control study (including 60 patients) (61).
Tissue factor pathway inhibitor (TFPI)
TFPI, a 276-amino acid Kunitz-type protease inhibitor, acts as the physiological modulator of the tissue factor (TF) pathway. TFPI mainly exerts its inhibitory effect on blood coagulation by inhibiting the TF-FVIIa complex in an FXa-dependent manner (62). TFPI is constitutively synthesized by endothelial cells and then conveyed to the endothelia (50–80%), plasma (20–50%) and platelets (2–5%) (63). Low plasma TFPI values have been observed in subjects with venous thrombosis, stroke and thrombotic thrombocytopenic purpura (64). Some studies showed that decreased plasma TFPI values may be present in subjects with both venous and arterial thrombotic disorders. Fei et al. showed that TFPI may be a valuable predictive biomarker of DVT and tumor metastasis, displaying high diagnostic sensitivity and specificity at the time of the diagnosis of non-small cell lung cancer (64). Zakai et al. confirmed that total TFPI concentration was higher in patients with high pro-coagulant factor values and increasing age. In particular, subjects with TFPI values <5th percentile were at moderately increased risk for VTE, after adjustment for other coagulation factors values (65). Irrespective of these findings, no clear evidence has been brought that TFPI deficiency should be considered a clinically significant prothrombotic condition.
Paroxysmal nocturnal haemoglobinuria (PNH)
PNH, also known as Marchiafava-Micheli syndrome, is a thrombophilic disorders due to an acquired mutation within the phosphatidyl-inositol glycan A (PIG-A) gene, which encodes for an enzyme involved in the biosynthesis of glycosylphosphatidylinositol (GPI)-anchor molecule (66). The typical clinical features of this condition also encompass complement-mediated intravascular hemolytic anemia and bone marrow failure (67). The deficient factors proteins entail also the complement regulatory proteins CD55 and CD59, which then lead to increased complement sensitivity of PNH cells, intravascular hemolysis, inflammation and systemic release of hemoglobin (68). Thromboembolism is the most frequent cause of mortality in PNH patients, accounting for approximately 40–67% of deaths. Although thrombosis may occur at any site in patients with PNH, venous thrombosis is reportedly more common than arterial complications. The most involved sites are intraabdominal and cerebral veins, whilst multiple sites can be involved in over 15% of cases. Hepatic vein thrombosis (also known as Budd-Chiari syndrome) is one of the most common sites of thrombosis, affecting 7.5–25% of PNH patients. Platelet activation, complement-mediated hemolysis, impaired bioavailability of nitric oxide (NO) and of the fibrinolytic system, along with inflammation, have been suggested as leading drivers of thrombosis in PNH (69). The diagnosis of PNH is mostly clinical, and should then be confirmed with flow cytometry studies, aimed at identifying the absence or severe deficiency of GPI-anchored proteins (GPI-APs) on ≥2 lineages (70). The lack or deficiency of GPI-APs can be identified with monoclonal antibodies and using the fluorescent aerolysin (FLAER) reagent (71).
Heparin-induced thrombocytopenia (HIT)
HIT is a rare but clinically important complication of heparin therapy sustained by an immune-mediated disorder due to immunoglobulins which bind to platelet factor 4 (PF4) (72). HIT is a clinicopathological syndrome, so that its diagnosis necessitates both clinical evaluation and laboratory investigations aimed at demonstrating the presence of specific antibodies in patient plasma, typically immunoglobulin G (IgG), directed against heparin-PF4 (H-PF4) complexes (73). The onset of HIT is associated with platelet activation and endothelial cell injury, which may then lead to disseminated thrombosis. The thrombotic complications develop in some HIT patients because platelets can be activated in vivo even when heparin has only been administered in the initial phase of antithrombotic prophylaxis (74). Several prospective and case-controlled studies showed that patients with HIT have a substantial risk of developing thrombosis, up to 35–75% in some selected clinical settings. The most common thrombotic event is represented by VTE, especially in postoperative patients, which may sometimes occur even at unusual vein site, such as cerebral or adrenal veins (72).
Conclusions
According to the current understanding that we have on the pathophysiology of thrombosis, a variety of unusual conditions may be effective to enhance the thrombotic risk in certain subjects over such a threshold that an acute thrombotic episode may then openly develop. Beside the rare thrombophilic disorders, which have been earlier discussed and should be clearly acknowledged to prevent or limit the burden of both venous and arterial thrombosis, some other “atypical triggers” have been described. These mostly include coughing or sneezing attacks, over-eating, migraine, defection, strenuous physical exercise or sexual intercourse, as well as abuse of drugs (75). Albeit some doubts remain on the true contribution that these less common factors may have on the risk of developing thrombosis (Table 1), we would be persuaded to agree with the famous Arthur Conan Doyle’s quote, that “when you have eliminated the impossible, whatever remains, however improbable, must be the truth”.
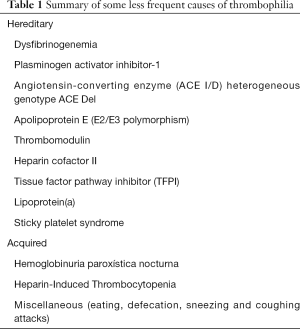
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lippi G, Favaloro EJ. Venous and Arterial Thromboses: Two Sides of the Same Coin? Semin Thromb Hemost 2018;44:239-48. [Crossref] [PubMed]
- Lippi G, Franchini M. Pathogenesis of venous thromboembolism: when the cup runneth over. Semin Thromb Hemost 2008;34:747-61. [Crossref] [PubMed]
- Grandone E, Tomaiuolo M, Colaizzo D, et al. Role of thrombophilia in adverse obstetric outcomes and their prevention using antithrombotic therapy. Semin Thromb Hemost 2009;35:630-43. [Crossref] [PubMed]
- Franchini M, Veneri D, Salvagno GL, et al. Inherited thrombophilia. Crit Rev Clin Lab Sci 2006;43:249-90. [Crossref] [PubMed]
- Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. J Thromb Haemost 2009;7 Suppl 1:301-4. [Crossref] [PubMed]
- Montagnana M, Lippi G, Danese E. An Overview of Thrombophilia and Associated Laboratory Testing. Methods Mol Biol 2017;1646:113-35. [Crossref] [PubMed]
- Lippi G, Danese E, Favaloro EJ, et al. Diagnostics in venous thromboembolism: from origin to future prospects. Semin Thromb Hemost 2015;41:374-81. [Crossref] [PubMed]
- Richter T, Nestler-Parr S, Babela R, et al. International Society for Pharmacoeconomics and Outcomes Research Rare Disease Special Interest Group. Rare Disease Terminology and Definitions-A Systematic Global Review: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2015;18:906-14. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Hemostasis practice: state-of-the-art. J Lab Precis Med 2018;3:67. [Crossref]
- de Moerloose P, Casini A, Neerman-Arbez M. Congenital fibrinogen disorders: an update. Semin Thromb Hemost 2013;39:585-95. [Crossref] [PubMed]
- Hayes T. Dysfibrinogenemia and thrombosis. Arch Pathol Lab Med 2002;126:1387-90. [PubMed]
- Korte W, Poon MC, Iorio A, et al. Thrombosis in Inherited Fibrinogen Disorders. Transfus Med Hemother 2017;44:70-6. [Crossref] [PubMed]
- Haverkate F, Samama M. Familial dysfibrinogenemia and thrombophilia. Report on a study of the SSC Subcommittee on Fibrinogen. Thromb Haemost 1995;73:151-61. [Crossref] [PubMed]
- Briginshaw GF, Shanberge JN. Identification of two distinct heparin cofactors in human plasma. Separation and partial purification. Arch Biochem Biophys 1974;161:683-90. [Crossref] [PubMed]
- Tollefsen DM. Heparin cofactor II modulates the response to vascular injury. Arterioscler Thromb Vasc Biol 2007;27:454-60. [Crossref] [PubMed]
- Kumar A, Bhandari A, Sarde SJ, et al. Genetic variants and evolutionary analyses of heparin cofactor II. Immunobiology 2014;219:713-28. [Crossref] [PubMed]
- Villa P, Aznar J, Vaya A, et al. Hereditary omozygous heparin cofactor II deficiency and the risk of developing venous thrombosis. Thromb Haemost 1999;82:1011-4. [Crossref] [PubMed]
- Lopaciuk S, Bykowska K, Kopeć M. Prevalence of heparin cofactor II deficiency in patients with a history of venous thrombosis. Pol J Pharmacol 1996;48:109-11. [PubMed]
- Ehrenforth S, Junker R, Koch HG, et al. Multicentre evaluation of combined prothrombotic defects associated with thrombophilia in childhood. Childhood Thrombophilia Study Group. Eur J Pediatr 1999;158:S97-104. [Crossref] [PubMed]
- Dahlbäck B, Villoutreix BO. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol 2005;25:1311-20. [Crossref] [PubMed]
- Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A 1981;78:2249-52. [Crossref] [PubMed]
- Anastasiou G, Gialeraki A, Merkouri E, et al. Thrombomodulin as a regulator of the anticoagulant pathway: implication in the development of thrombosis. Blood Coagul Fibrinolysis 2012;23:1-10. [Crossref] [PubMed]
- Koutsi A, Papapanagiotou A, Papavassiliou AG. Thrombomodulin: from haemostasis to inflammation and tumourigenesis. Int J Biochem Cell Biol 2008;40:1669-73. [Crossref] [PubMed]
- Kumada T, Dittman WA, Majerus PW. A role for thrombomodulin in the pathogenesis of thrombin-induced thromboembolism in mice. Blood 1988;71:728-33. [PubMed]
- Gomi K, Zushi M, Honda G, et al. Antithrombotic effect of recombinant human thrombomodulin on thrombin-induced thromboembolism in mice. Blood 1990;75:1396-9. [PubMed]
- Weiler-Guettler H, Christie PD, Beeler DL, et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest 1998;101:1983-91. [Crossref] [PubMed]
- Isermann B, Hendrickson SB, Zogg M, et al. Endothelium-specific loss of murine thrombomodulin disrupts the protein C anticoagulant pathway and causes juvenile-onset thrombosis. J Clin Invest 2001;108:537-46. [Crossref] [PubMed]
- Ahmad A, Sundquist K, Zöller B, et al. Thrombomodulin gene c.1418C>T polymorphism and risk of recurrent venous thromboembolism. J Thromb Thrombolysis 2016;42:135-41. [Crossref] [PubMed]
- Heit JA, Petterson TM, Owen WG, et al. Thrombomodulin gene polymorphisms or haplotypes as potential risk factors for venous thromboembolism: a population-based case-control study. J Thromb Haemost 2005;3:710-7. [Crossref] [PubMed]
- Le Flem L, Picard V, Emmerich J, et al. Mutations in promoter region of thrombomodulin and venous thromboembolic disease. Arterioscler Thromb Vasc Biol 1999;19:1098-104. [Crossref] [PubMed]
- Lippi G, Guidi G. Lipoprotein(a): an emerging cardiovascular risk factor. Crit Rev Clin Lab Sci 2003;40:1-42. [Crossref] [PubMed]
- Lippi G, Targher G. Optimal therapy for reduction of lipoprotein(a). J Clin Pharm Ther 2012;37:1-3. [Crossref] [PubMed]
- Lippi G, Targher G, Franchini M. Lipoprotein(a), thrombophilia and venous thrombosis. Acta Haematol 2007;117:246-7. [Crossref] [PubMed]
- Lippi G, Guidi G. Lipoprotein(a): from ancestral benefit to modern pathogen? QJM 2000;93:75-84. [Crossref] [PubMed]
- Dentali F, Gessi V, Marcucci R, et al. Lipoprotein(a) as a Risk Factor for Venous Thromboembolism: A Systematic Review and Meta-analysis of the Literature. Semin Thromb Hemost 2017;43:614-20. [Crossref] [PubMed]
- Lippi G, Franchini M, Targher G. Screening and therapeutic management of lipoprotein(a) excess: review of the epidemiological evidence, guidelines and recommendations. Clin Chim Acta 2011;412:797-801. [Crossref] [PubMed]
- Seman LJ, DeLuca C, Jenner JL, et al. Lipoprotein(a)-cholesterol and coronary heart disease in the Framingham Heart Study. Clin Chem 1999;45:1039-46. [PubMed]
- Seed M, Ayres KL, Humphries SE, et al. Lipoprotein (a) as a predictor of myocardial infarction in middle-aged men. Am J Med 2001;110:22-7. [Crossref] [PubMed]
- Sofi F, Marcucci R, Abbate R, et al. Lipoprotein (a) and venous thromboembolism in adults: a meta-analysis. Am J Med 2007;120:728-33. [Crossref] [PubMed]
- Helgadottir A, Gretarsdottir S, Thorleifsson G, et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol 2012;60:722-9. [Crossref] [PubMed]
- Rubenfire M, Blevins RD, Barnhart M, et al. Platelet hyperaggregability in patients with chest pain and angiographically normal coronary arteries. Am J Cardiol 1986;57:657-60. [Crossref] [PubMed]
- Sokol J, Skerenova M, Jedinakova Z, et al. Progress in the Understanding of Sticky Platelet Syndrome. Semin Thromb Hemost 2017;43:8-13. [PubMed]
- Tekgündüz E, Demir M, Akyol Erikçi A, et al. Sticky platelet syndrome in patients with uninduced venous thrombosis. Turk J Haematol 2013;30:48-52. [PubMed]
- Solis-Jimenez F, Hinojosa-Heredia H, García-Covarrubias L, et al. Sticky Platelet Syndrome: An Unrecognized Cause of Acute Thrombosis and Graft Loss. Case Rep Nephrol 2018;2018. [Crossref] [PubMed]
- Kubisz P, Stanciakova L, Stasko J, et al. Sticky platelet syndrome: an important cause of life-threatening thrombotic complications. Expert Rev Hematol 2016;9:21-35. [Crossref] [PubMed]
- Yamamoto K, Takeshita K, Saito H. Plasminogen activator inhibitor-1 in aging. Semin Thromb Hemost 2014;40:652-9. [Crossref] [PubMed]
- Cale JM, Lawrence DA. Structure-function relationships of plasminogen activator inhibitor-1 and its potential as a therapeutic agent. Curr Drug Targets 2007;8:971-81. [Crossref] [PubMed]
- Asselbergs FW, Pattin K, Snieder H, et al. Genetic architecture of tissue-type plasminogen activator and plasminogen activator inhibitor-1. Semin Thromb Hemost 2008;34:562-8. [Crossref] [PubMed]
- Flevaris P, Vaughan D. The Role of Plasminogen Activator Inhibitor Type-1 in Fibrosis. Semin Thromb Hemost 2017;43:169-77. [Crossref] [PubMed]
- Sundquist K, Wang X, Svensson PJ, et al. Plasminogen activator inhibitor-1 4G/5G polymorphism, factor V Leiden, prothrombin mutations and the risk of VTE recurrence. Thromb Haemost 2015;114:1156-64. [Crossref] [PubMed]
- Tsantes AE, Nikolopoulos GK, Bagos PG, et al. Association between the plasminogen activator inhibitor-1 4G/5G polymorphism and venous thrombosis. A meta-analysis. Thromb Haemost 2007;97:907-13. [Crossref] [PubMed]
- Tsantes AE, Nikolopoulos GK, Bagos PG, et al. The effect of the plasminogen activator inhibitor-1 4G/5G polymorphism on the thrombotic risk. Thromb Res 2008;122:736-42. [Crossref] [PubMed]
- Huebbe P, Rimbach G. Evolution of human apolipoprotein E (APOE) isoforms: Gene structure, protein function and interaction with dietary factors. Ageing Res Rev 2017;37:146-61. [Crossref] [PubMed]
- Phillips MC. Apolipoprotein E isoforms and lipoprotein metabolism. IUBMB Life 2014;66:616-23. [Crossref] [PubMed]
- Zhu S, Wang Z, Wu X, et al. Apolipoprotein E polymorphism is associated with lower extremity deep venous thrombosis: color-flow Doppler ultrasound evaluation. Lipids Health Dis 2014;13:21. [Crossref] [PubMed]
- Ghebranious N, Ivacic L, Mallum J, et al. Detection of ApoE E2, E3 and E4 alleles using MALDI-TOF mass spectrometry and the homogeneous mass-extend technology. Nucleic Acids Res 2005;33. [Crossref] [PubMed]
- Stoumpos S, Hamodrakas SJ, Anthopoulos PG, et al. The association between apolipoprotein E gene polymorphisms and essential hypertension: a meta-analysis of 45 studies including 13,940 cases and 16,364 controls. J Hum Hypertens 2013;27:245-55. [Crossref] [PubMed]
- Wang QY, Wang WJ, Wu L, et al. Meta-analysis of APOE ε2/ε3/ε4 polymorphism and cerebral infarction. J Neural Transm (Vienna) 2013;120:1479-89. [Crossref] [PubMed]
- Rubino E, Vacca A, Govone F, et al. Apolipoprotein E polymorphisms in frontotemporal lobar degeneration: a meta-analysis. Alzheimers Dement 2013;9:706-13. [Crossref] [PubMed]
- Yin YW, Li JC, Wang JZ, et al. Association between apolipoprotein E gene polymorphism and the risk of vascular dementia: a meta-analysis. Neurosci Lett 2012;514:6-11. [Crossref] [PubMed]
- Nagato LC, de Souza Pinhel MA, de Godoy JM, et al. Association of ApoE genetic polymorphisms with proximal deep venous thrombosis. J Thromb Thrombolysis 2012;33:116-9. [Crossref] [PubMed]
- Adams M. Tissue factor pathway inhibitor: new insights into an old inhibitor. Semin Thromb Hemost 2012;38:129-34. [Crossref] [PubMed]
- Maroney SA, Haberichter SL, Friese P, et al. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood 2007;109:1931-7. [Crossref] [PubMed]
- Fei X, Wang H, Yuan W, et al. Tissue Factor Pathway Inhibitor-1 Is a Valuable Marker for the Prediction of Deep Venous Thrombosis and Tumor Metastasis in Patients with Lung Cancer. Biomed Res Int 2017;2017. [Crossref] [PubMed]
- Zakai NA, Lutsey PL, Folsom AR, et al. Total tissue factor pathway inhibitor and venous thrombosis. The Longitudinal Investigation of Thromboembolism Etiology. Thromb Haemost 2010;104:207-12. [Crossref] [PubMed]
- Schubert J, Röth A. Update on paroxysmal nocturnal haemoglobinuria: on the long way to understand the principles of the disease. Eur J Haematol 2015;94:464-73. [Crossref] [PubMed]
- Marotta S, Pagliuca S, Risitano AM. Hematopoietic stem cell transplantation for aplastic anemia and paroxysmal nocturnal hemoglobinuria: current evidence and recommendations. Expert Rev Hematol 2014;7:775-89. [Crossref] [PubMed]
- Holguin MH, Fredrick LR, Bernshaw NJ, et al. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest 1989;84:7-17. [Crossref] [PubMed]
- Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 2013;121:4985-96. [Crossref] [PubMed]
- Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood 2009;113:6522-7. [Crossref] [PubMed]
- Brodsky RA, Mukhina GL, Li S, et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol 2000;114:459-66. [Crossref] [PubMed]
- Warkentin TE. New approaches to the diagnosis of heparin-induced thrombocytopenia. Chest 2005;127:35S-45S. [Crossref] [PubMed]
- Tan CW, Ward CM, Morel-Kopp MC. Evaluating heparin-induced thrombocytopenia: the old and the new. Semin Thromb Hemost 2012;38:135-43. [Crossref] [PubMed]
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995;332:1330-5. [Crossref] [PubMed]
- Lippi G, Franchini M, Favaloro EJ. Unsuspected triggers of venous thromboembolism--trivial or not so trivial? Semin Thromb Hemost 2009;35:597-604. [Crossref] [PubMed]


