Diagnostic insights into chronic-inflammatory demyelinating polyneuropathies
Introduction
There has been remarkable progress in the clinical and electrophysiological categorization of acute and chronic immune-mediated neuropathies recently. However, the serological diagnosis of chronic inflammatory demyelinating polyneuropathies (CIDP) is still inconsistent and the search for useful serological markers is ongoing (1,2). CIDP represents a rare disabling autoimmune disorder of peripheral nervous system, with poorly understood etiopathogenesis. Various incidences have been reported, ranging from 0.8 to 8.9 per 100,000 individuals per year depending on geographical origin of the patient cohorts investigated (3). Nevertheless, along with acute polyneuropathies classified as the Guillain-Barré syndrome (GBS) CIDP accounts for the majority of immune mediated polyneuropathies (4).
Once correctly diagnosed, several causal treatment options are available for a large part of the CIDP patients, with satisfactory success rates (5,6). As second line therapy options, biologicals (e.g., rituximab), immunosuppressant or immunomodulatory drugs may be considered when patients do not respond adequately to plasmapheresis or intravenous immunoglobulin (IVIg) (7). The diagnosis of CIDP is mainly based on clinical and electrophysiological criteria (8). Typical clinical symptoms of CIDP comprise symmetrical, proximal and/or distal paresis as well as sensory loss and develop over a period of at least 8 weeks (9). Hereditary neuropathies which should be taken into consideration for differential diagnosis of CIDP variants will be not covered in this review.
Several diagnostic criteria with differing sensitivities have been discussed recently (10). Altogether, the diagnostic criteria of the European Federation of Neurological Sciences (EFNS) established in cooperation with the Peripheral Nerve Society (PNS) and refined in 2010 (11) have gained widespread acceptance (8). Thus, CIDP can be classified into typical CIDP and atypical variants such as distal acquired-demyelinating polyneuropathy (DADS), multifocal-acquired demyelinating sensory and motor polyneuropathy (MADSAM) also referred to as Lewis-Sumner syndrome, and acute-onset CIDP (A-CIDP) (12-14). Due to acute onset and, thus, the similarity of the clinical phenotype with acute immune-mediated neuropathies such as the GBS, the diagnosis of A-CIDP can be delayed (15). In contrast, DADS as an atypical variant is often associated with a monoclonal gammopathy and, hence, sometimes difficult to differentiate from paraproteinemic neuropathies such as chronic sensory ataxic neuropathy with IgM autoantibodies (autoAbs) to disialosyl gangliosides also referred to as CANOMAD (chronic ataxic neuropathy, ophthalmoplegia, IgM paraprotein, cold agglutinin and antidisialosyl antibodies) (16,17).
Altogether, the defined diagnostic criteria of EFNS/PNS permit a broad range of clinical variants to be grouped under the clinical entity CIDP. However, these variants might be characterized by different pathogenic mechanisms. Novel markers could aid in stratification of patients with CIDP, in order to address the diversity of clinical phenotype and response to treatment of typical and various atypical CIDP variants. Several laboratory abnormalities were reported for CIDP patients such as paraproteinemia, elevated hemoglobulin A1c and creatinine kinase, as well as positive vasculitic neuropathy markers (1). Notwithstanding, neither of these laboratory abnormalities were specific for CIDP and could be considered as diagnostic criteria as it is the case for acute immune-mediated neuropathy) (18).
Pathophysiology of CIDP
The leading pathogenic process in CIDP is the multifocal demyelination of nerve cells affecting nerve roots, plexus and fibers as well as conditions mimicking this process (19-21). The latter refer to an emerging concept based on electrophysiological and experimental findings demonstrating a conduction failure with typical “axonal” damage characteristics which, however, can rapidly recover (reversible conduction failure) (20).
Experimental evidence on passive and active animal transfer models, active immunization with nerve components and response to immunosuppressive treatment, IVIg as well as plasmapheresis, suggest that dysfunctional acquired immune responses may play a pivotal role in the pathogenesis of CIDP (2,22-26). In this context, the heterogeneous clinical manifestation of CIDP may hint at pathophysiological processes involving humoral autoimmune responses against differing nerve fiber components. As a fact, IgG and IgM as well as complement deposits were demonstrated in patients with chronic inflammatory neuropathies (27). Moreover, compared to normal controls, one study reported increased serum levels of anaphylatoxin C5a and terminal complement complex (C5b9) in serum and cerebrospinal fluid (CSF) of CIDP patients (28). Autoreactive T-cell responses against myelin epitopes have also been reported, which lends further evidence to a certain role of a tolerance break to distinct components of the peripheral nerve system (26,29). Furthermore, CD4+ and particularly CD8+ T cells were identified in inflammatory infiltrates of patients with CIDP (30,31). Last but not least, elevated levels of inflammatory cytokines such as interleukin 2, interleukin 6, tumor necrosis factor alpha and B-cell activating factor were reported in serum and CSF of CIDP patients (32-35).
Altogether, there is mounting evidence that an autoimmune attack against distinct components of peripheral nerves particularly of the node and paranode regions is very likely as leading pathogenic mechanism. Likely, this autoimmune attack is triggered by microbial molecular mimicry (36). Hence, it is not surprising that multiple novel autoAbs identified recently have been proposed as potential biomarkers for CIDP (2,37-39). Nevertheless, it should be mentioned that no serum marker is recognized to be diagnostic currently despite the clear correlation of certain autoAbs with distinct peripheral neuropathy variants (40,41).
Diagnostic options in CIDP
The diagnosis of CIPD relies on observation of neurological clinical symptoms of demyelination and detection of demyelinating electrophysiological features, as well as elevated CSF protein levels (8,42,43). New conduction studies may aid in the discrimination of demyelination (conduction block or reduced conduction velocity) and axonal impairment (diminished compound muscle action potential amplitude). The latter is, in general, accompanied with poor prognosis, but may rapidly recover. This is seen in patients with nodo-paranodopathies, a new concept in the diagnosis of autoimmune mediated polyneuropathies (21).
Clinical impairment is recommended to be assessed by the Medical Research Council (MRC) (44) and the inflammatory neuropathy cause and treatment (INCAT) disability score (45). Furthermore, disease activity may be ascertained by the Clinical Disease Activity Status (CDAS) with the classification in unstable and stable stages (46).
When a diagnosis cannot be established by the former features, biopsy of the nerve affected with assessment of inflammatory infiltrates may provide additional helpful information. However, inflammatory infiltrates may not be detectable at all, or only occasionally, which mirrors the heterogeneous clinical picture of CIDP (47). Thus, characteristic signatures of de- (thin myelin sheath around large axons) or re-myelination (onion bulbs) and endoneuronal edema should be considered as further biopsy characteristics (48).
Recently, non-invasive imaging techniques such as magnet resonance imaging of nerve roots and fibers or sonography have been successfully utilized in clinical studies as additional diagnostic options to support a diagnosis of CIDP (49-51). Furthermore, interesting diagnostic results have been achieved by corneal confocal microscopy due to the association of CIDP with small fiber damage (52,53).
Nevertheless, the diagnosis of CIDP remains challenging and it is occasionally confirmed by the response to a causal therapy only (8). Misdiagnosis of CIDP with inappropriate therapy was reported in up to 47% of CIDP patients investigated (54). Thus, the early diagnosis of CIDP and treatment initiation is essential for preventing irreversible axonal damage and disability. Hence, the search for additional biomarkers in particular serological ones continues (2). Serological markers could help supporting an early diagnosis. In addition, such biomarkers could assist in predicting treatment response and differentiating between clinical phenotypes.
AutoAbs as potential markers in CIDP
AutoAbs to nerve components were reported to play a pathogenic role in acute autoimmune peripheral neuropathies such as GBS (55-57). As a fact, autoAbs to glycoconjugate molecules like gangliosides or the myelin-associated glycoprotein (MAG) have gained widespread use in serological work-up of patients with acute peripheral neuropathies (56). In this context, the use of assay technique has been a contentious debate regarding the optimal epitope presentation for correct autoAb analysis (37,58-61). Interestingly, multiplex assay techniques such as line immunoassays (LIA), glycoarrays, and flow cytometry evolved as novel promising diagnostic tools to address clinical needs (58,62-64). In contrast to acute peripheral neuropathies, the role of autoAb testing in CIDP is still elusive (5). This is astonishing to a certain extend given the plethora of data indicating a pathogenic role of autoimmune responses in CIDP. Increasing evidence indicates that autoAbs to targets involved in saltatory conduction at the nodes of Ranvier and adjacent regions may represent marker candidates (65). The autoimmune attack of these autoAbs can mimic demyelination and present with a reverse conduction block, also referred to as axonal conduction block based on disruption of nodal axolemma (4).
AutoAbs to nodal and paranodal targets could be ascertained by the use of tissue-based fluorescence assays revealing in up to 30% of patients with immune-mediated neuropathies including CIDP such autoAbs (66). These findings sparked the intensive search for the corresponding targets responsible for specific autoAb binding. Hence, the diagnostic role of autoAbs to distinct targets related to the node of Ranvier and adjacent regions as well as to non-regional related components reported in CIDP so far (Table 1) and their corresponding detection techniques should be in the focus of this review.
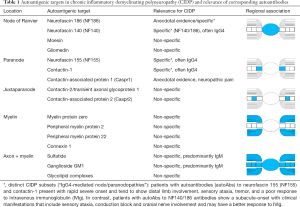
Full table
AutoAbs to specific nerve fiber regions
AutoAbs to nodal targets
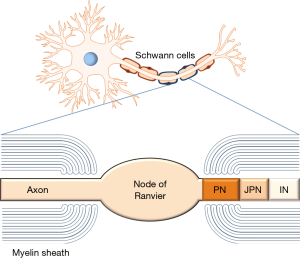
Potential nodal autoantigenic targets investigated in CIDP have been neurofascin (NF) 186, moesin, and gliomedin (67,68) (Table 1). Gliomedin is a microvilli cell adhesion molecule of Schwann cells interacting with NF186 of the axon. In turn, NF 186 is linked along with other molecules to the voltage-gated sodium channels enriched in the nodal region and responsible for inward current of action and saltatory conduction finally (69). Consequently, the lack of NF186 interferes with axonal conduction, as elegantly demonstrated in NF186 null mice (70). Remarkably, autoAbs against the nodal neurofascin NF186 have been found in CIDP (66). Recently, autoAbs to NF140/186 (mainly IgG4) targeting epitopes different from autoAbs against NF155 and specific for a subset of CIDP showing subacute-onset and include sensory ataxia, conduction block and cranial nerve involvement have been found (39). Nevertheless, autoAbs to paranodal targets, in particular of the IgG4 isotype, seem to be more frequent in CIDP and may help in stratifying patients with CIDP variants (4).
AutoAbs to paranodal targets
Paranodes fence the internodal region and prevent the diffusion of nodal molecules like NF186 and voltage-gated sodium channels to that region (71). Furthermore, the integrity of the paranode is important to prevent interruption by juxtaparanodal voltage-gated potassium channels (72,73). Paranodal autoAbs against NF155 have been found consistently in CIDP patients with combined central and peripheral demyelination (CCPD) (74,75) and in a subset of CIDP patients with distinct clinical features (76) (Table 1). Out of the other molecules forming septate-like junctions in the paranodal region such as contactin-1 (CNTN1) and contactin-associated protein (Caspr), CNTN1 seems to be another relevant autoantigenic target in CIDP (65). The presence of particularly IgG4 to CNTN1 and NF155 was confirmed by several other clinical evaluations recently demonstrating an aggressive disease onset and poor responsiveness to IVIgs (68,77-80). Furthermore, Querol and coworkers found only paranodal autoAbs against NF155 and CNTN1 to be specific markers in CIDP (2). Both autoAbs seem to be pathogenic by interfering with NF155/CNTN1 complex in a complement-independent manner, which has also implications for treatment decisions (68).
AutoAbs to juxtaparanodal targets
The potential role of autoAbs to juxtaparanodal targets such as CNTN2 also referred to as transient axonal glycoprotein 1 (TAG1) and Caspr2 interfering with the stability of the voltage-gated potassium channel complex is an emerging hypothesis (4,81). Loss of tolerance against these potential targets has not been conclusively reported so far. Interestingly, an association of distinct single nucleotide polymorphisms of TAG1 with the responsiveness of CIDP patients to IVIg therapy is discussed controversially (82,83).
AutoAbs to non-regional related components
AutoAbs to myelin proteins
Despite extensive studies on the potential role of myelin proteins (i.e., myelin protein zero, peripheral myelin protein 2 or 22, and connexin 1) as autoimmune targets in CIDP, no significant associations of corresponding autoAbs with CIDP could be established (2,29,84-86) (Table 1). This was confirmed by a compelling study using indirect immunofluorescence on various cellular substrates and immunoprecipitation (2). In contrast, autoAbs to MAG were reported in patients with DADS (16).
AutoAbs to gangliosides/sulfatide
Unlike acute immune-mediated neuropathies, the value of autoAb testing to gangliosides and sulfatide has been still illusive in chronic immune-mediated polyneuropathies, and only established for a minority of them (Table 1). Thus, IgM autoAbs against disialosyl epitopes, particularly to GD1b, were found in chronic sensory ataxic neuropathy demonstrating often similar clinical features of CIDP (17). Furthermore, patients suffering from the CANOMAD syndrome demonstrated IgM autoAbs to the disialosyl gangliosides GD1b, GD3, GT1b, and GQ1b (17). Most patients with IgM autoAbs against GD1b profited from IVIg therapy or biologicals (87,88). These IgM autoAbs appeared to be pathogenic in terms of sensory ataxia, which can also be observed in CIDP.
Furthermore, autoAbs to sulfatide, which is predominantly expressed within the non-compact myelin, were associated with different subtypes of peripheral neuropathy, most of them axonal (60,89). However, a demyelinating type with a lower prevalence was also described (90). In acute polyneuropathies, a particular strong association of pathogenic autoAbs with distinct clinical variants [such as autoAbs against GQ1b to the Miller-Fisher syndrome (MFS), a subtype of the GBS], could be ascertained (37,91,92). Conversely, in terms of chronic immune-mediated neuropathies, IgM autoAbs against GM1 were reported in up to 60% of patients with multifocal motor neuropathy (MMN), a progressively worsening pure motor polyneuropathy (93-95). Of note, increased titers of IgM autoAbs to sulfatide were detected in patients with neuropathy, where they are often associated with a concomitant reactivity to the MAG (96). In contrast, Giannotta and coworkers reported reactivity to sulfatide in only 1% of CIDP patients (97). Furthermore, a recent retrospective analysis found IgM autoAbs to GM1 in 46% of patients with MMN but in only 3% of CIDP patients (93).
In a recent study, an elevated frequency of at least one IgM autoAb to GM1, GD1b and, sulfatide in patients suffering from CIDP was reported (98). Remarkably, patients positive for autoAbs to sulfatide were younger and showed typical manifestations of clinical symptoms of CIDP but no association with axonal degeneration and neither any association with monoclonal IgM gammopathy nor with positivity of autoAbs to MAG reported earlier (90,96,97,99). Of note, cerebroside sulfotransferase-deficient mice demonstrated paranodal disruption by juxtaparanodal voltage-gated potassium channel invasion which underscores the role of sulfatide in stabilizing the paranodal junctions (100). Furthermore, autoAbs to sulfatide-ganglioside complexes detected by a combinatorial glycoarray methodology accounted for the largest group of antiglycolipid autoAbs in patients with GBS (60). Thus, the assay technique used for the analysis of such autoAbs appears to play a pivotal role. Thin-layer chromatography is supposed to be the gold-standard assay technique for the assessment of antiglycolipid autoAbs, though it is not applicable for routine use (63). Methods such as the LIA or the combinatorial glycoarray may be a good alternative for the multiplex assessment of autoAbs to gangliosides and sulfatide due to an optimal autoantigenic epitope-preserving binding on hydrophobic polyvinylidene difluoride membranes (64,101). The hydrophobic solid phase has already proven its usefulness for the specific analysis of auto/Abs to amphipathic molecules like lipopolysaccharides and phospholipids exhibiting similar physicochemical characteristics (102-105). In the context of antiphospholipid antibody testing, hydrophobic membranes appear to result in a better assay performance than for instance solid phases used in enzyme-linked immunosorbent assays (106-108).
Altogether, differences in assay techniques could be the reason for differing reports on the frequency of autoAbs to gangliosides and sulfatide (97). Thus, higher frequencies of IgM autoAbs to GM1 (16%) detected by LIA were found in CIDP and MMN patients in contrast to the glycoarray (7%), where IgM to glycolipid complexes containing GM1 and sulfatide were the most frequently observed autoAbs in CIDP patients (98,109). Interestingly, the patients of both studies demonstrated motor disturbances more frequently than autoAb-negative ones did. Moreover, patients with positivity of autoAbs to sulfatide showed a higher rate of conduction blocks in nerve conduction studies (98). These findings add further evidence to the assumption that impairment of primarily motor functions in CIDP may be explained by depletion of sulfatide and myelin proteins such as neurofascin 155 especially in the paranodal region (89). Furthermore, the ganglioside GM1 is highly expressed on the membranes of motor nerves and on the surface of Schwann cells. Binding of autoAbs to these targets on the axon at the nodes of Ranvier or on Schwann cells (see Figure 1) may cause complement activation and disruption of sodium channel clusters resulting in conduction abnormalities (57,110).
Clinical relevance of autoAbs against paranodal proteins
Since its first description in 1958 (111) results of numerous studies, case series and case reports indicate that CIDP is not a defined disease entity but rather a spectrum of related chronic neuromuscular disorders. The phenotypic variability and response to therapy may be driven by different pathomechanisms that are associated with autoantigenic targets of immune responses (19). Therefore, autoAbs specific for defined CIDP subtypes may be helpful in their early diagnosis leading to the most effective therapy. Although numerous autoAbs have been described in CIDP, only IgG4 autoAbs against paranodal proteins (i.e., neurofascin 155, contactin 1, Caspr1) determined by cell-based assays or ELISA using human native autoantigens showed a very high specificity for a defined clinical phenotype named “autoimmune nodo-paranodopathy” (2,15,112).
AutoAbs against neurofascin 155: summarizing the 12 studies which tested autoAbs against NF155 by using native human NF155, the overall frequency was 6.4% (90/1,404), with predominant IgG4 response in CIDP patients (66). The frequency differs between the studies from 4% to 18% (38,73,75,76,79,113-115). These studies, along with that of Siles et al. (116), showed a very high diagnostic specificity (>99–100%) by testing of more than 200 blood donors and 1,109 patients with other neurological diseases including GBS, MFS, multiple sclerosis (MS), MMN, paraneoplastic neurological syndromes, MAG antibody-positive and genetic neuropathies. Only some GBS patients (frequency <1%) were found positive with a predominant IgG1 or IgM response (38,73,75,76,79,113,115). Although the clinical picture may vary slightly among studies, a specific clinical phenotype that differs from the autoAb-negative CIDP has been described, which includes a younger age of onset, a subacute and more severe onset, disabling tremor, sensory and cerebellar ataxia, distal dominant weakness, and poor response to IVIg (75,76,79,113). Furthermore, an association of NF155 autoAb with CCPD has been described in Japanese but not in Caucasian patients (66,73).
AutoAbs to CNTN1 with predominant IgG4 isotype were found in 3–8% of CIDP patients, with a diagnostic specificity of 100% vs. blood donors, GBS, and MMN (38,66,78). Patients with autoAbs to CNTN1 show a special clinical phenotype, including a more advanced age of onset compared to autoAb negative CIDP, an aggressive and GBS-like subacute onset of weakness, a very high ratio of sensory ataxia, early axonal involvement, and poor response to IVIg (66).
AutoAbs against Caspr1: up to now, autoAbs against Caspr1 were described in two studies only, showing a cumulative frequency in CIDP patients of about 1% (3/281) and a high diagnostic specificity (66). These were only detectable in one out of 48 GBS patients, but none of 52 MS patients, 32 patients with Charcot-Marie-Tooth disease, 34 patients with possible or definite paraneoplastic neurological syndromes and 78 blood donors (38,116,117). Whilst the GBS patient had IgG3 autoAb, the autoAb to Caspr1 of the CIDP patient in the study of Doppler et al. was of the IgG4 isotype. This patient had a subacute, severe, motor dominant onset, severe pain, reversible conduction block, was unresponsive to IVIg and corticosteroids, but showed a good response to B cell depletion (117).
Taken together, CIDP positive for autoAbs against the paranodal proteins NF155, CNTN1, and Caspr1 represent a different CIDP subtype (autoimmune nodo-paranodopathy) compared to seronegative CIDP with poor response to IVIG therapy, but partial favorable steroid and plasmapheresis responses (66). Therefore, IVIG is not a primary therapeutic option, especially in patients with autoAbs to NF155. First studies demonstrated that most seropositive CIDP patients had a good response to rituximab, a B cell depleting therapy (66,115,117,118). In conclusion, autoAbs against paranodal proteins should be determined for an early diagnosis of autoimmune nodo-paranodopathies indicating the treatment with rituximab.
Summary
The diagnosis of CIDP and its variants is based on clinical and electrophysiological features. Emerging autoAbs, especially against paranodal cell-adhesion molecules such as NF155, CNTN1, and Caspr1 as well as to glycolipids (gangliosides and sulfatide) appear to be good marker candidates for CIDP subentities, i.e., may aid in discriminating the diverse clinical variants and/or the response to treatment. AutoAbs to NF155 and Caspr1 of the immunoglobulin subtype IgG4 appear to be associated with a poor response to IVIg therapy, but good response to B cell depletion. On the other site, autoAbs to NF140/186 may be associated with a better response to IVIg.
Acknowledgements
None.
Footnote
Conflicts of Interest: D Roggenbuck has a management role and is a shareholder of GA Generic Assays GmbH and Medipan GmbH. Both companies are diagnostic manufacturers. The other authors have no conflicts of interest to declare.
References
- Abraham A, Albulaihe H, Alabdali M, et al. Frequent laboratory abnormalities in CIDP patients. Muscle Nerve 2016;53:862-5. [Crossref] [PubMed]
- Querol L, Siles AM, Alba-Rovira R, et al. Antibodies against peripheral nerve antigens in chronic inflammatory demyelinating polyradiculoneuropathy. Sci Rep 2017;7:14411. [Crossref] [PubMed]
- Mahdi-Rogers M, Hughes RA. Epidemiology of chronic inflammatory neuropathies in southeast England. Eur J Neurol 2014;21:28-33. [Crossref] [PubMed]
- Fehmi J, Scherer SS, Willison HJ, et al. Nodes, paranodes and neuropathies. J Neurol Neurosurg Psychiatry 2018;89:61-71. [Crossref] [PubMed]
- Dalakas MC. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol 2011;7:507-17. [Crossref] [PubMed]
- Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol 2008;7:136-44. [Crossref] [PubMed]
- Kleyman I, Brannagan TH 3rd. Treatment of chronic inflammatory demyelinating polyneuropathy. Curr Neurol Neurosci Rep 2015;15:47. [Crossref] [PubMed]
- Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur J Neurol 2010;17:356-63. [Crossref] [PubMed]
- Viala K, Maisonobe T, Stojkovic T, et al. A current view of the diagnosis, clinical variants, response to treatment and prognosis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst 2010;15:50-6. [Crossref] [PubMed]
- Breiner A, Brannagan TH 3rd. Comparison of sensitivity and specificity among 15 criteria for chronic inflammatory demyelinating polyneuropathy. Muscle Nerve 2014;50:40-6. [Crossref] [PubMed]
- Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. J Peripher Nerv Syst 2005;10:220-8. [Crossref] [PubMed]
- Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat Rev Neurol 2014;10:435-46. [Crossref] [PubMed]
- Eftimov F, van Schaik I. Chronic inflammatory demyelinating polyradiculoneuropathy: update on clinical features, phenotypes and treatment options. Curr Opin Neurol 2013;26:496-502. [Crossref] [PubMed]
- Viala K, Renie L, Maisonobe T, et al. Follow-up study and response to treatment in 23 patients with Lewis-Sumner syndrome. Brain 2004;127:2010-7. [Crossref] [PubMed]
- Ruts L, Drenthen J, Jacobs BC, et al. Distinguishing acute-onset CIDP from fluctuating Guillain-Barre syndrome: a prospective study. Neurology 2010;74:1680-6. [Crossref] [PubMed]
- Katz JS, Saperstein DS, Gronseth G, et al. Distal acquired demyelinating symmetric neuropathy. Neurology 2000;54:615-20. [Crossref] [PubMed]
- Willison HJ, O'Leary CP, Veitch J, et al. The clinical and laboratory features of chronic sensory ataxic neuropathy with anti-disialosyl IgM antibodies. Brain 2001;124:1968-77. [Crossref] [PubMed]
- Van der Meché FG, Van Doorn PA, Meulstee J, et al. Diagnostic and classification criteria for the Guillain-Barre syndrome. Eur Neurol 2001;45:133-9. [Crossref] [PubMed]
- Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry 2015;86:973-85. [Crossref] [PubMed]
- Uncini A, Yuki N. Sensory Guillain-Barre syndrome and related disorders: an attempt at systematization. Muscle Nerve 2012;45:464-70. [Crossref] [PubMed]
- Uncini A, Kuwabara S. Nodopathies of the peripheral nerve: an emerging concept. J Neurol Neurosurg Psychiatry 2015;86:1186-95. [Crossref] [PubMed]
- Saida T, Saida K, Dorfman SH, et al. Experimental allergic neuritis induced by sensitization with galactocerebroside. Science 1979;204:1103-6. [Crossref] [PubMed]
- Van der Meché FG, Hartung HP, Kieseier BC. From bench to bedside--experimental rationale for immune-specific therapies in the inflamed peripheral nerve. Nat Clin Pract Neurol 2007;3:198-211. [Crossref] [PubMed]
- Salomon B, Rhee L, Bour-Jordan H, et al. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J Exp Med 2001;194:677-84. [Crossref] [PubMed]
- Yan WX, Taylor J, Andrias-Kauba S, et al. Passive transfer of demyelination by serum or IgG from chronic inflammatory demyelinating polyneuropathy patients. Ann Neurol 2000;47:765-75. [Crossref] [PubMed]
- Klehmet J, Goehler J, Ulm L, et al. Effective treatment with intravenous immunoglobulins reduces autoreactive T-cell response in patients with CIDP. J Neurol Neurosurg Psychiatry 2015;86:686-91. [Crossref] [PubMed]
- Dalakas MC, Engel WK. Immunoglobulin and complement deposits in nerves of patients with chronic relapsing polyneuropathy. Arch Neurol 1980;37:637-40. [Crossref] [PubMed]
- Quast I, Keller CW, Hiepe F, et al. Terminal complement activation is increased and associated with disease severity in CIDP. Ann Clin Transl Neurol 2016;3:730-5. [Crossref] [PubMed]
- Csurhes PA, Sullivan AA, Green K, et al. T cell reactivity to P0, P2, PMP-22, and myelin basic protein in patients with Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry 2005;76:1431-9. [Crossref] [PubMed]
- Yang M, Peyret C, Shi XQ, et al. Evidence from Human and Animal Studies: Pathological Roles of CD8(+) T Cells in Autoimmune Peripheral Neuropathies. Front Immunol 2015;6:532. [Crossref] [PubMed]
- Schneider-Hohendorf T, Schwab N, Uceyler N, et al. CD8+ T-cell immunity in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 2012;78:402-8. [Crossref] [PubMed]
- Ritter C, Forster D, Albrecht P, et al. IVIG regulates BAFF expression in patients with chronic inflammatory demyelinating polyneuropathy (CIDP). J Neuroimmunol 2014;274:225-9. [Crossref] [PubMed]
- Hartung HP, Reiners K, Schmidt B, et al. Serum interleukin-2 concentrations in Guillain-Barre syndrome and chronic idiopathic demyelinating polyradiculoneuropathy: comparison with other neurological diseases of presumed immunopathogenesis. Ann Neurol 1991;30:48-53. [Crossref] [PubMed]
- Maimone D, Annunziata P, Simone IL, et al. Interleukin-6 levels in the cerebrospinal fluid and serum of patients with Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Neuroimmunol 1993;47:55-61. [Crossref] [PubMed]
- Sainaghi PP, Collimedaglia L, Alciato F, et al. The expression pattern of inflammatory mediators in cerebrospinal fluid differentiates Guillain-Barre syndrome from chronic inflammatory demyelinating polyneuropathy. Cytokine 2010;51:138-43. [Crossref] [PubMed]
- Yuki N. Ganglioside mimicry and peripheral nerve disease. Muscle Nerve 2007;35:691-711. [Crossref] [PubMed]
- Goodfellow JA, Willison HJ. Antiganglioside, antiganglioside-complex, and antiglycolipid-complex antibodies in immune-mediated neuropathies. Curr Opin Neurol 2016;29:572-80. [Crossref] [PubMed]
- Querol L, Devaux J, Rojas-Garcia R, et al. Autoantibodies in chronic inflammatory neuropathies: diagnostic and therapeutic implications. Nat Rev Neurol 2017;13:533-47. [Crossref] [PubMed]
- Delmont E, Manso C, Querol L, et al. Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain 2017;140:1851-8. [Crossref] [PubMed]
- Rinaldi S, Willison HJ. Ganglioside antibodies and neuropathies. Curr Opin Neurol 2008;21:540-6. [Crossref] [PubMed]
- Willison HJ. Gangliosides as targets for autoimmune injury to the nervous system. J Neurochem 2007;103 Suppl 1:143-9. [Crossref] [PubMed]
- Dyck PJ, Lais AC, Ohta M, et al. Chronic inflammatory polyradiculoneuropathy. Mayo Clin Proc 1975;50:621-37. [PubMed]
- Bouchard C, Lacroix C, Plante V, et al. Clinicopathologic findings and prognosis of chronic inflammatory demyelinating polyneuropathy. Neurology 1999;52:498-503. [Crossref] [PubMed]
- Kleyweg RP, van der Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve 1991;14:1103-9. [Crossref] [PubMed]
- Merkies IS, Schmitz PI, Van Der Meche FG, et al. Psychometric evaluation of a new handicap scale in immune-mediated polyneuropathies. Muscle Nerve 2002;25:370-7. [Crossref] [PubMed]
- Gorson KC, van Schaik IN, Merkies IS, et al. Chronic inflammatory demyelinating polyneuropathy disease activity status: recommendations for clinical research standards and use in clinical practice. J Peripher Nerv Syst 2010;15:326-33. [Crossref] [PubMed]
- Vital A, Lagueny A, Julien J, et al. Chronic inflammatory demyelinating polyneuropathy associated with dysglobulinemia: a peripheral nerve biopsy study in 18 cases. Acta Neuropathol 2000;100:63-8. [Crossref] [PubMed]
- Webster HD, Schroder JM, Asbury AK, et al. The role of Schwann cells in the formation of "onion bulbs" found in chronic neuropathies. J Neuropathol Exp Neurol 1967;26:276-99. [Crossref] [PubMed]
- Shibuya K, Sugiyama A, Ito S, et al. Reconstruction magnetic resonance neurography in chronic inflammatory demyelinating polyneuropathy. Ann Neurol 2015;77:333-7. [Crossref] [PubMed]
- Kerasnoudis A, Pitarokoili K, Behrendt V, et al. Bochum ultrasound score versus clinical and electrophysiological parameters in distinguishing acute-onset chronic from acute inflammatory demyelinating polyneuropathy. Muscle Nerve 2015;51:846-52. [Crossref] [PubMed]
- Kerasnoudis A, Pitarokoili K, Behrendt V, et al. Nerve ultrasound score in distinguishing chronic from acute inflammatory demyelinating polyneuropathy. Clin Neurophysiol 2014;125:635-41. [Crossref] [PubMed]
- Bucher F, Schneider C, Blau T, et al. Small-Fiber Neuropathy Is Associated With Corneal Nerve and Dendritic Cell Alterations: An In Vivo Confocal Microscopy Study. Cornea 2015;34:1114-9. [Crossref] [PubMed]
- Stettner M, Hinrichs L, Guthoff R, et al. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol 2015;3:88-100. [Crossref] [PubMed]
- Allen JA, Lewis RA. CIDP diagnostic pitfalls and perception of treatment benefit. Neurology 2015;85:498-504. [Crossref] [PubMed]
- Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain 2002;125:2591-625. [Crossref] [PubMed]
- Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet 2016;388:717-27. [Crossref] [PubMed]
- Susuki K, Rasband MN, Tohyama K, et al. Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers. J Neurosci 2007;27:3956-67. [Crossref] [PubMed]
- Escande-Beillard N, David MJ, Portoukalian J, et al. A sensitive flow cytometry method for anti-GM1 antibodies detection. J Neuroimmunol 2002;125:163-9. [Crossref] [PubMed]
- Caudie C, Quittard Pinon A, Bouhour F, et al. Comparison of commercial tests for detecting multiple anti-ganglioside autoantibodies in patients with well-characterized immune-mediated peripheral neuropathies. Clin Lab 2013;59:1277-87. [Crossref] [PubMed]
- Rinaldi S, Brennan KM, Kalna G, et al. Antibodies to heteromeric glycolipid complexes in Guillain-Barre syndrome. PLoS One 2013;8. [Crossref] [PubMed]
- Delmont E, Robb H, Davidson A, et al. Prospective study comparing enzyme-linked immunosorbent assay and glycoarray assay to detect antiglycolipid antibodies in a routine diagnostic neuroimmunology laboratory setting. Clin Exp Neuroimmunol 2015;6:175-82. [Crossref]
- Galban-Horcajo F, Halstead SK, McGonigal R, et al. The application of glycosphingolipid arrays to autoantibody detection in neuroimmunological disorders. Curr Opin Chem Biol 2014;18:78-86. [Crossref] [PubMed]
- Chabraoui F, Derrington EA, Mallie-Didier F, et al. Dot-blot immunodetection of antibodies against GM1 and other gangliosides on PVDF-P membranes. J Immunol Methods 1993;165:225-30. [Crossref] [PubMed]
- Conrad K, Schneider H, Ziemssen T, et al. A new line immunoassay for the multiparametric detection of antiganglioside autoantibodies in patients with autoimmune peripheral neuropathies. Ann N Y Acad Sci 2007;1109:256-64. [Crossref] [PubMed]
- Stathopoulos P, Alexopoulos H, Dalakas MC. Autoimmune antigenic targets at the node of Ranvier in demyelinating disorders. Nat Rev Neurol 2015;11:143-56. [Crossref] [PubMed]
- Devaux JJ, Odaka M, Yuki N. Nodal proteins are target antigens in Guillain-Barre syndrome. J Peripher Nerv Syst 2012;17:62-71. [Crossref] [PubMed]
- Vural A, Doppler K, Meinl E. Autoantibodies Against the Node of Ranvier in Seropositive Chronic Inflammatory Demyelinating Polyneuropathy: Diagnostic, Pathogenic, and Therapeutic Relevance. Front Immunol 2018;9:1029. [Crossref] [PubMed]
- Dalakas MC, Gooch C. Close to the node but far enough: What nodal antibodies tell us about CIDP and its therapies. Neurology 2016;86:796-7. [Crossref] [PubMed]
- Rasband MN. Composition, assembly, and maintenance of excitable membrane domains in myelinated axons. Semin Cell Dev Biol 2011;22:178-84. [Crossref] [PubMed]
- Thaxton C, Pillai AM, Pribisko AL, et al. Nodes of Ranvier act as barriers to restrict invasion of flanking paranodal domains in myelinated axons. Neuron 2011;69:244-57. [Crossref] [PubMed]
- Amor V, Zhang C, Vainshtein A, et al. The paranodal cytoskeleton clusters Na(+) channels at nodes of Ranvier. Elife 2017;6. [Crossref] [PubMed]
- Pillai AM, Thaxton C, Pribisko AL, et al. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J Neurosci Res 2009;87:1773-93. [Crossref] [PubMed]
- Susuki K, Baba H, Tohyama K, et al. Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers. Glia 2007;55:746-57. [Crossref] [PubMed]
- Kawamura N, Yamasaki R, Yonekawa T, et al. Anti-neurofascin antibody in patients with combined central and peripheral demyelination. Neurology 2013;81:714-22. [Crossref] [PubMed]
- Ng JK, Malotka J, Kawakami N, et al. Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology 2012;79:2241-8. [Crossref] [PubMed]
- Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology 2014;82:879-86. [Crossref] [PubMed]
- Devaux JJ, Miura Y, Fukami Y, et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology 2016;86:800-7. [Crossref] [PubMed]
- Miura Y, Devaux JJ, Fukami Y, et al. Contactin 1 IgG4 associates to chronic inflammatory demyelinating polyneuropathy with sensory ataxia. Brain 2015;138:1484-91. [Crossref] [PubMed]
- Doppler K, Appeltshauser L, Wilhelmi K, et al. Destruction of paranodal architecture in inflammatory neuropathy with anti-contactin-1 autoantibodies. J Neurol Neurosurg Psychiatry 2015;86:720-8. [Crossref] [PubMed]
- Ogata H, Yamasaki R, Hiwatashi A, et al. Characterization of IgG4 anti-neurofascin 155 antibody-positive polyneuropathy. Ann Clin Transl Neurol 2015;2:960-71. [Crossref] [PubMed]
- Poliak S, Salomon D, Elhanany H, et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol 2003;162:1149-60. [Crossref] [PubMed]
- Pang SY, Chan KH, Mak WW, et al. Single-nucleotide polymorphism of transient axonal glycoprotein-1 and its correlation with clinical features and prognosis in chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst 2012;17:72-5. [Crossref] [PubMed]
- Uncini A, Manzoli C, Notturno F, et al. Pitfalls in electrodiagnosis of Guillain-Barre syndrome subtypes. J Neurol Neurosurg Psychiatry 2010;81:1157-63. [Crossref] [PubMed]
- Yan WX, Archelos JJ, Hartung HP, et al. P0 protein is a target antigen in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol 2001;50:286-92. [Crossref] [PubMed]
- Kwa MS, van Schaik IN, Brand A, et al. Investigation of serum response to PMP22, connexin 32 and P(0) in inflammatory neuropathies. J Neuroimmunol 2001;116:220-5. [Crossref] [PubMed]
- Inglis HR, Csurhes PA, McCombe PA. Antibody responses to peptides of peripheral nerve myelin proteins P0 and P2 in patients with inflammatory demyelinating neuropathy. J Neurol Neurosurg Psychiatry 2007;78:419-22. [Crossref] [PubMed]
- Attarian S, Boucraut J, Hubert AM, et al. Chronic ataxic neuropathies associated with anti-GD1b IgM antibodies: response to IVIg therapy. J Neurol Neurosurg Psychiatry 2010;81:61-4. [Crossref] [PubMed]
- Delmont E, Jeandel PY, Hubert AM, et al. Successful treatment with rituximab of one patient with CANOMAD neuropathy. J Neurol 2010;257:655-7. [Crossref] [PubMed]
- Palavicini JP, Wang C, Chen L, et al. Novel molecular insights into the critical role of sulfatide in myelin maintenance/function. J Neurochem 2016;139:40-54. [Crossref] [PubMed]
- Dabby R, Weimer LH, Hays AP, et al. Antisulfatide antibodies in neuropathy: clinical and electrophysiologic correlates. Neurology 2000;54:1448-52. [Crossref] [PubMed]
- Chiba A, Kusunoki S, Shimizu T, et al. Serum IgG antibody to ganglioside GQ1b is a possible marker of Miller Fisher syndrome. Ann Neurol 1992;31:677-9. [Crossref] [PubMed]
- Plomp JJ, Molenaar PC, O'Hanlon GM, et al. Miller Fisher anti-GQ1b antibodies: alpha-latrotoxin-like effects on motor end plates. Ann Neurol 1999;45:189-99. [Crossref] [PubMed]
- Nobile-Orazio E, Giannotta C, Briani C. Anti-ganglioside complex IgM antibodies in multifocal motor neuropathy and chronic immune-mediated neuropathies. J Neuroimmunol 2010;219:119-22. [Crossref] [PubMed]
- Nobile-Orazio E, Giannotta C, Musset L, et al. Sensitivity and predictive value of anti-GM1/galactocerebroside IgM antibodies in multifocal motor neuropathy. J Neurol Neurosurg Psychiatry 2014;85:754-8. [Crossref] [PubMed]
- Kornberg AJ, Pestronk A. Chronic motor neuropathies: diagnosis, therapy, and pathogenesis. Ann Neurol 1995;37 Suppl 1:S43-50. [Crossref] [PubMed]
- Campagnolo M, Ferrari S, Dalla TC, et al. Polyneuropathy with anti-sulfatide and anti-MAG antibodies: clinical, neurophysiological, pathological features and response to treatment. J Neuroimmunol 2015;281:1-4. [Crossref] [PubMed]
- Giannotta C, Di Pietro D, Gallia F, et al. Anti-sulfatide IgM antibodies in peripheral neuropathy: to test or not to test? Eur J Neurol 2015;22:879-82. [Crossref] [PubMed]
- Klehmet J, Märschenz S, Ruprecht K, et al. Analysis of anti-ganglioside antibodies by a line immunoassay in patients with chronic-inflammatory demyelinating polyneuropathies (CIDP). Clin Chem Lab Med 2018;56:919-26. [Crossref] [PubMed]
- Fraussen J, Claes N, de Bock L, et al. Targets of the humoral autoimmune response in multiple sclerosis. Autoimmun Rev 2014;13:1126-37. [Crossref] [PubMed]
- Ishibashi T, Dupree JL, Ikenaka K, et al. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J Neurosci 2002;22:6507-14. [Crossref] [PubMed]
- Caudie C, Quittard Pinon A, Bouhour F, et al. Comparison of commercial tests for detecting multiple anti-ganglioside autoantibodies in patients with well-characterized immune-mediated peripheral neuropathies. Clin Lab 2013;59:1277-87. [Crossref] [PubMed]
- Seifert M, Schoenherr G, Roggenbuck D, et al. Generation and characterization of a human monoclonal IgM antibody that recognizes a conserved epitope shared by lipopolysaccharides of different gram-negative bacteria. Hybridoma 1996;15:191-8. [Crossref] [PubMed]
- Schoenherr G, Roggenbuck D, Seifert M, et al. Technical problems arising from the use of the immunoblot for determination of the reactivity of natural antibodies with different lipopolysaccharides (LPS). J Immunol Methods 1996;190:185-8. [Crossref] [PubMed]
- Roggenbuck D, Borghi MO, Somma V, et al. Antiphospholipid antibodies detected by line immunoassay differentiate among patients with antiphospholipid syndrome, with infections and asymptomatic carriers. Arthritis Res Ther 2016;18:111. [Crossref] [PubMed]
- Nalli C, Somma V, Andreoli L, et al. Anti-phospholipid IgG antibodies detected by line immunoassay differentiate patients with anti-phospholipid syndrome and other autoimmune diseases. Auto Immun Highlights 2018;9:6. [Crossref] [PubMed]
- Roggenbuck D, Egerer K, von Landenberg P, et al. Antiphospholipid antibody profiling - Time for a new technical approach. Autoimmun Rev 2012;11:821-6. [Crossref] [PubMed]
- Roggenbuck D, Somma V, Schierack P, et al. Autoantibody profiling in APS. Lupus 2014;23:1262-4. [Crossref] [PubMed]
- Egerer K, Roggenbuck D, Buettner T, et al. Single-step autoantibody profiling in antiphospholipid syndrome using a multi-line dot assay. Arthritis Res Ther 2011;13:R118. [Crossref] [PubMed]
- Morikawa M, Kuwahara M, Ueno R, et al. Serological study using glycoarray for detecting antibodies to glycolipids and glycolipid complexes in immune-mediated neuropathies. J Neuroimmunol 2016;301:35-40. [Crossref] [PubMed]
- Cats EA, Jacobs BC, Yuki N, et al. Multifocal motor neuropathy: association of anti-GM1 IgM antibodies with clinical features. Neurology 2010;75:1961-7. [Crossref] [PubMed]
- Austin JH. Recurrent polyneuropathies and their corticosteroid treatment; with five-year observations of a placebo-controlled case treated with corticotrophin, cortisone, and prednisone. Brain 1958;81:157-92. [Crossref] [PubMed]
- Uncini A, Vallat JM. Autoimmune nodo-paranodopathies of peripheral nerve: the concept is gaining ground. J Neurol Neurosurg Psychiatry 2018;89:627-35. [Crossref] [PubMed]
- Kadoya M, Kaida K, Koike H, et al. IgG4 anti-neurofascin155 antibodies in chronic inflammatory demyelinating polyradiculoneuropathy: Clinical significance and diagnostic utility of a conventional assay. J Neuroimmunol 2016;301:16-22. [Crossref] [PubMed]
- Mathey EK, Garg N, Park SB, et al. Autoantibody responses to nodal and paranodal antigens in chronic inflammatory neuropathies. J Neuroimmunol 2017;309:41-6. [Crossref] [PubMed]
- Burnor E, Yang L, Zhou H, et al. Neurofascin antibodies in autoimmune, genetic, and idiopathic neuropathies. Neurology 2018;90:e31-8. [Crossref] [PubMed]
- Siles AM, Martinez-Hernandez E, Araque J, et al. Antibodies against cell adhesion molecules and neural structures in paraneoplastic neuropathies. Ann Clin Transl Neurol 2018;5:559-69. [Crossref] [PubMed]
- Doppler K, Appeltshauser L, Villmann C, et al. Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathy. Brain 2016;139:2617-30. [Crossref] [PubMed]
- Querol L, Rojas-Garcia R, Diaz-Manera J, et al. Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflamm 2015;2. [Crossref] [PubMed]


