Thyroid stimulating hormone and free triiodothyronine are valuable predictors for diabetic nephropathy in patient with type 2 diabetes mellitus
Introduction
As the third leading cause of mortality, diabetes mellitus is prevalent and seriously threatens human health worldwide (1). There are many serious complications of diabetes such as microvascular complications and kidney impairment (2,3). Nephropathy is the main complication of diabetes which could lead to kidney failure (4). Diabetic nephropathy (DN) is a progressive deterioration in kidney function, represented by augmented glomerular filtration rate (GFR), glomerular hypertrophy, and urinary leakage of albumin (5). Moreover, DN is associated with poor outcomes, and is a unique predictor of mortality in both insulin dependent and independent diabetes (6,7). Therefore, early identification of diabetes and microvascular complications risk provides an opportunity to introduce preventive interventions to stop or delay disease onset (8), which would be more important to decrease the morbidity and mortality of type 2 diabetes mellitus (T2DM) patients.
It was shown that many biological markers or biomarkers can reflect the presence of microvascular damage in T2DM patients (9,10), and are associated with the risk of microangiopathy and developing T2DM (11-13). It has been recognized that many biomarkers play potential roles in the diagnosis and prognosis of DN (14). Elevated thyroid stimulating hormone (TSH) and reduced T3 could be commonly observed in diabetes patients (15). TSH level is reported to interact with the renal disease progression and development of malignancy (16,17). The low T3 level is associated with inflammation in chronic kidney disease (CKD) patients (18), indicating its potential role in the development of the decline of renal function. However, there were little studies focus on the relationship between thyroid hormones levels and risk of DN in patients with T2DM. Therefore, this study aimed to explore the predicting significance of thyroid hormones for DN.
Methods
Patients population
A total of 301 patients with T2DM were finally enrolled. Patients included 191 males and 110 females aged 37–85 (median: 61) years from the Department of Endocrinology, Zhejiang Provincial People’s Hospital, China, between October 2015 and December 2016. All patients with T2DM and DN were firstly diagnosed according to the criteria in diabetes guideline 2013 of China Diabetes Association (19). The baseline data of patients before treatment included clinical history, risk factors, physical examination, clinical and biologic data during hospitalization, and routine laboratory tests. For all patients, the inclusion criteria included: (I) diabetes without complications; (II) diabetes with nephropathy; (III) before treatment for elevated glucose, high blood pressure, dyslipidemia, etc.; and the exclusion criteria included: (I) other diabetic complications; (II) primary liver and kidney dysfunctions; (III) postoperation; (IV) hypertension; (V) cardiovascular and cerebrovascular diseases; (VI) malignancies; (VII) acute inflammation and infections; (VIII) positive anti-thyroid antibody; (IX) using drugs altering thyroid hormone concentrations such as beta-blockers, amiodarone, corticosteroids, etc. T2DM patients were grouped into T2DM without complications (202 patients) and T2DM with nephropathy (99 patients). For comparison, 101 healthy controls [age, gender, race and body mass index (BMI)-matched] were included in the study. The controls were recruited from the Health Management Center of the hospitals, and had normal levels of fasting serum glucose and glycosylated hemoglobin, and did not take any drugs in two weeks before samples collecting.
Laboratory assay
Venous blood of patients was collected in the morning after an overnight fast for measurements of thyroid hormones including total triiodothyronine (T3), free triiodothyronine (FT3), total thyroxine (T4), free thyroxine (FT4), and TSH before treatment, respectively. For thyroid hormones analysis, 3 mL blood sample was collected into vacutainer tubes (Becton Dickinson, MountainView, CA, USA) without any anticoagulant. Sera were obtained by centrifugation at 1,500 g at room temperature for 10 minutes. Subsequently, the levels of T3, FT3, T4, FT4 and TSH were measured with a chemiluminescent analyzer and the commercial reagents (DXI800, Beckman-Coulter, USA) within four hours after sample collection, respectively. Routine biochemical parameters (including serum creatine, cystatin C, fasting serum glucose, Peptide-C, glycosylated hemoglobin, serum high-density lipoprotein, total cholesterol, and albumin) were measured. Estimated glomerular filtration rate (eGFR) was calculated according to the formula based on the CKD-EPIscr_cys equation (20). The biological and clinical data of all patients (age and gender, diabetic complications, T2DM-related and pathological data) before treatment were collected and reviewed.
Statistical analysis
One-way analysis of variance (ANOVA) was first used to analyze the difference among control, T2DM without complications, and DN groups, then the difference was analyzed by SNK-test. For samples of the normal distribution (thyroid hormones levels) and not normal (age) data, Student’s t-test and Mann-Whitney U-test were used, respectively. Chi-square test was used for categorical variables (gender). Multivariate Logistic regression analysis using the actual numerical values of thyroid hormone concentrations was performed to calculate the odds ratio and its 95% confidence interval for T2DM and DN. Receiver operating characteristic (ROC) curve was constructed, and area under curves was calculated to evaluate the predicting power of the independent risk factors. Statistical analyses were performed using statistical package SPSS 20.0 (SPSS, Chicago, Illinois, USA). P value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics of patients
From October 2015 to December 2016, 301 consecutive patients with T2DM confirmed diagnosis were enrolled in this study. Median age at diagnosis is 61 years old, ranging from 37 to 85 years, where male patients constituted the majority of the group (n=193, 64%). Detailed baseline clinical and biological characteristics of patients are presented in Table 1.
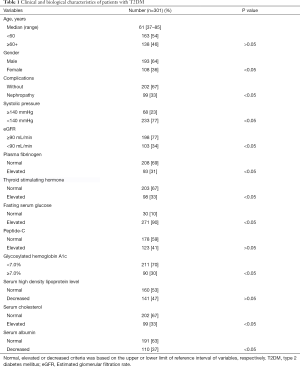
Full table
Comparisons of thyroid hormones levels between patients with T2DM and healthy controls
There was no statistical difference in any investigated variable between controls and patients without complications (P>0.05). DN patients demonstrated lower T3 and FT3 levels, but higher TSH level than those in controls and patients without complications (P<0.001 for all variables). There was no significant difference for other variables between the three groups. Detailed data were presented in Table 2.

Full table
Results of regression analysis of thyroid hormones for T2DM and DN
The association of each parameter with the risk for T2DM and DN was analyzed separately. Multivariate analysis was performed in controls and patients without complications, and the results revealed that all the variables were not independently correlated with T2DM without complications. Further multivariate analysis in all T2DM patients with DN and without complications demonstrated that increased TSH and decreased T3 and FT3 as the independent predicting factors for patients with DN [odds ratio (OR): 2.087, 95% CI: 1.525–3.303; 1.335, 95% CI: 1.101–1.621; 7.414, 95% CI: 4.319–13.986; P<0.001, respectively]. Detailed data were presented in Table 3.

Full table
Results of ROC curves analysis of the predicting factors for DN
ROC curve was used to evaluate the predicting power of the three factors for DN. For T2DM with DN, the area under ROC curve demonstrated the predicting power of TSH, FT3 and T3 (0.850, 95% CI: 0.776–0.923; 0.824, 95% CI: 0.751–0.897, and 0.620, 95% CI: 0.515–0.725; respectively). Based on their cutoff values of 1.85 mIU/L, 2.31 ng/L, and 0.61 µg/L, the sensitivity was 82%, 78%, and 64%, and the specificity was 77%, 79%, and 85%, respectively. Data were presented in Figure 1 and Table 4.
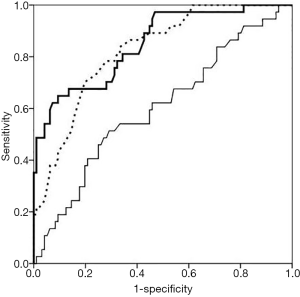
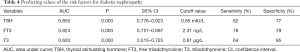
Full table
Discussion
It has been proved that various biomarkers, including hematology, biochemistry, immunology, hemostasis, would be influenced in the development of diabetes mellitus (DM) (21) Around 2.2–17% diabetic patients were reported being comorbid with thyroid dysfunction, among which the hypothyroidism is the commonest (22), especially women (23). In this study, we found that there were no significantly different thyroid hormones levels in complication-free T2DM compared with controls, which was not consistent with the results of hypothyroidism in some studies (22,24). However, the thyroid function profiles vary between reports among DN patients (24,25). Our study revealed that T3, FT3, and TSH would be useful predictors for DN, and it was necessary to explore further whether they were valuable predictors for DN.
Although our study did not exhibit abnormal levels of thyroid hormones in patients without complications, decreased levels of thyroid hormones were found in DN patients. In recent years, various studies have demonstrated that diabetes and diabetic microvascular disease patients, especially DN, would exhibit hypothyroidism (22,24,26). Therefore, further study was performed to evaluate the significance of thyroid hormones in predicting T2DM and DN. Multivariate regression analysis showed that no thyroid hormones had high OR value in predicting T2DM. At the same time, T3, FT3 and TSH exhibited high OR values in predicting DN. The results indicated that decreased T3 and FT3, and increased TSH might imply the risk for DN. Therefore, the study also further revealed that thyroid function assays might be valuable for monitoring the occurrence of DN. To further assess the predicting power of the three risk factors mentioned above, ROC curve analysis was used. Although T3 showed higher specificity (83%) in the ROC curve, the area under curve (AUC) (0.620) did not confirm its predictive value in the real-world practice. However, TSH and FT3 exhibited the AUC of 0.850 and 0.824 in prediction for DN with the cutoff of 0.85 mIU/L and 2.31 ng/L, respectively. The results revealed that TSH and FT3 had high predicting power than that of T3 for DN in T2DM patients. T2DM patients with increased TSH or decreased FT3 level may be at risk for DN, which indicates that it is useful for TSH and FT3 to predict DN in T2DM patients.
There are at least two limitations concerning the present study. Firstly, there probably were some nephropathy patients with other complications, and T2DM patients with complications which were not diagnosed before treatment. Therefore, the laboratory data from those patients would cause some uncertain results, and might potentially influence the predicting power. Secondly, percent of patients with advanced DN also might potentially increase the predicting power, and patients were not stratified based on disease condition of nephropathy in this study. Therefore, it was unclear whether there was a significant difference for levels of thyroid hormones between early- and end-stages of DN. Although there were the limitations, the present study also revealed the valuable predicting significance of TSH and FT3 in prediction for DN in T2DM patients.
Conclusions
This study suggests that TSH and FT3 are useful predictors for DN in patients with T2DM. However, further multiple centers and controlled prospective studies on large groups of patients may give more definite results.
Acknowledgments
The authors thank all those who supported them in patients’ recruitment and help them to gather patients information.
Funding: This work was supported by the Natural Science Foundation of Zhejiang Province (Grant No. LY17H080007 to X Fei).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (ID of ethics approval: 2015KY073). The study outcomes will not affect the future management of the patients. The use of human blood samples was in accordance with the legislation in China. Informed consent was obtained from the controls and patients or their relatives, and the study was approved by the institutional review board of the three hospitals.
References
- Tabák AG, Jokela M, Akbaraly TN, et al. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009;373:2215-21. [Crossref] [PubMed]
- Liu X, Li Y, Li L, et al. Prevalence, awareness, treatment, control of type 2 diabetes mellitus and risk factors in Chinese rural population: the Rural Diab study. Sci Rep 2016;6:31426. [Crossref] [PubMed]
- Li S, Yu C, Li Y, et al. Study design and baseline characteristics of inpatients with diabetes mellitus in a tertiary hospital in China: A database study based on electronic medical records. J Evid Based Med 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Zhang X, Cui X, Li F, et al. Association between diabetes mellitus with metabolic syndrome and diabetic microangiopathy. Exp Ther Med 2014;8:1867-73. [Crossref] [PubMed]
- Nordquist L, Wahren J. C-Peptide: The missing link in diabetic nephropathy? Rev Diabet Stud 2009;6:203-10. [Crossref] [PubMed]
- Shlipak M. Diabetic nephropathy. BMJ Clin Evid 2009;2009.
- Menzies RI, Booth J, Mullins JJ, et al. Hyperglycemia-induced renal P2X7 receptor activation enhances diabetes-related injury. EBioMedicine 2017;19:73-83. [Crossref] [PubMed]
- Abbasi A, Peelen LM, Corpeleijn E, et al. Prediction models for risk of developing type 2 diabetes: systematic literature search and independent external validation study. BMJ 2012;345. [Crossref] [PubMed]
- Ho H, Cheung CY, Sabanayagam C, et al. Retinopathy signs improved prediction and reclassification of cardiovascular disease risk in diabetes: A prospective cohort study. Sci Rep 2017;7:41492. [Crossref] [PubMed]
- Li S, Wei J, Zhang C, et al. Cell-Derived Microparticles in Patients with Type 2 Diabetes Mellitus: a Systematic Review and Meta-Analysis. Cell Physiol Biochem 2016;39:2439-50. [Crossref] [PubMed]
- Toth PP, Simko RJ, Palli SR, et al. The impact of serum lipids on risk for microangiopathy in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2012;11:109. [Crossref] [PubMed]
- Chakrabarti S, Khan ZA, Cukiernik M, et al. C-peptide and retinal microangiopathy in diabetes. Exp Diabesity Res 2004;5:91-6. [Crossref] [PubMed]
- Zheng N, Shi X, Chen X, et al. Associations between inflammatory markers, hemostatic markers, and microvascular complications in 182 Chinese patients with type 2 diabetes mellitus. Lab Med 2015;46:214-20. [Crossref] [PubMed]
- Raj DS, Pecoits-Filho R, Kimmel PL. Inflammation in chronic kidney disease. In: Kimmel PL, Rosenberg ME. editors. Chronic Renal Disease. Cambridge: Academic Press, 2015:199-212.
- Yagura T, Ishii H, Yoshimasa T, et al. Multivariable analysis of serum 3,5,3'-L-triiodothyronine concentration in patients of diabetes mellitus by blood glucose level and body weight. Horm Metab Res 1990;22:237-40. [Crossref] [PubMed]
- Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab 2012;16:204-13. [Crossref] [PubMed]
- Jia Q, Li X, Liu Y, et al. Incidental thyroid carcinoma in surgery-treated hyperthyroid patients with Graves' disease: a systematic review and meta-analysis of cohort studies. Cancer Manag Res 2018;10:1201-7. [Crossref] [PubMed]
- Zoccali C, Tripepi G, Cutrupi S, et al. Low triiodothyronine: A new facet of inflammation in end-stage renal disease. J Am Soc Nephrol 2005;16:2789-95. [Crossref] [PubMed]
- China Diabetes Association. China Diabetes prevention and Treatment Guideline 2013. Chin J Diabete 2014;22:2-42.
- Chi XH, Li GP, Wang QS, et al. CKD-EPI creatinine-cystatin C glomerular filtration rate estimation equation seems more suitable for Chinese patients with chronic kidney disease than other equations. BMC Nephrol 2017;18:226. [Crossref] [PubMed]
- Gloyn AL, Drucker DJ. Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endocrinol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Rai S, Kumar JA, Prajna K, et al. Thyroid function in type 2 diabetes mellitus and in diabetic nephropathy. J Clin Diagn Res 2013;7:1583-5. [PubMed]
- Papazafiropoulou A, Sotiropoulos A, Kokolaki A, et al. Prevalence of thyroid dysfunction among Greek type 2 diabetic patients attending an outpatient clinic. J Clin Med Res 2010;2:75-8. [PubMed]
- Wu J, Li X, Tao Y, et al. Free Triiodothyronine levels are associated with diabetic nephropathy in euthyroid patients with type 2 diabetes. Int J Endocrinol 2015;2015. [Crossref] [PubMed]
- Vadivelan M, Sahoo J, Bobby Z, et al. Thyroid dysfunction in patients with type 2 diabetes mellitus and its association with diabetic complications. J Assoc Physicians India 2016;64:91-2. [PubMed]
- Qi Q, Zhang QM, Li CJ, et al. Association of thyroid-stimulating hormone levels with microvascular complications in type2 diabetes patients. Med Sci Monit 2017;23:2715-20. [Crossref] [PubMed]


