Sleep-disordered breathing in paediatric setting: existing and upcoming of the genetic disorders
Introduction
Obstructive sleep apnea (OSA) is the consequence of a partial or complete intermittent cessation of airflow and bring on recurrent oxygen desaturations during sleep (1). The severity of OSA depends, in large part, from the patency of the high airway (2). In children, the major contributor to high airway obstruction is hyperplasia of pharyngeal tonsils and adenoids; craniofacial disharmony is also largely associated (3). Most studies showed a prevalence of sleep-disordered breathing (SDB) between 1% and 4% in children (4).
Polysomnography (PSG) is suggested for children with snoring and symptoms/signs of obstructive sleep apnea syndrome (OSAS) (5). If PSG is not available, alternative diagnostic tests (polygraph, pulsossimetry) (6), or referral to a specialized centre for evaluation, ought to be considered (7). The apnoea-hypopnoea index (AHI) is the sum of apneas (obstructive—OA and central apneas—CA) plus hypopneas per hour of sleep (episodes/hr). Obstructive AHI (oAHI) is the sum of OA plus hypopneas per hour of sleep (n./hr). Children with an AHI of >5 episodes/hr, those with an AHI of 1–5 episodes/hr, and morbidity or factors predicting OSA persistence, and children with complex conditions (genetic syndromes affecting the high-airways morphology) require appropriate management (8), ranging from treatment that alters underlying conditions contributing to OSAS (weight loss, anti-inflammatory treatment, myofunctional therapy) to orthodontic treatment (rapid maxillary expansion—RME), upper airway surgery (i.e., adenotonsillectomy—A&T, mandibular distraction osteogenesis), nasal continuous positive airway pressure (CPAP) or nasal noninvasive positive pressure ventilation (NPPV), depending upon the severity of the condition (9).
The present review is aimed to update the recent findings regarding OSAS in pediatric genetic diseases, recommended on research journals and international guidelines (achondroplasia—ACH, Down syndrome—DS, Prader-Willi syndrome—PWS, Pierre Robin sequence—PRS, Sickle cell disease—SSD) (10,11) or encountered in our clinical practice (Ehlers-Danlos syndrome—EDS, Ellis-van Creveld syndrome—EVC, Noonan syndrome—NS) (Table 1). Two additional groups of genetic disorders will be focused (mucopolysaccharidoses—MPS and osteogenesis imperfecta—OI). The flowing items are covered on these genetic diseases: (I) what is the pathophysiology of SDB? (II) What is the incidence/prevalence of SDB? (III) What is the outcome of the management and prognosis? (IV) What are the recommendations?
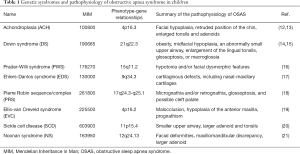
Full table
What is the pathophysiology of OSAS in genetic syndromes?
Achondroplasia
ACH is a disorder of bone growth that involves the changing of cartilage to bone (particularly of the long bones of the arms and legs). ACH is characterized by an increased risk of SDB (22-25). These patients have macrocephaly and facial hypoplasia, dysplasia of the skull base and foramen magnum stenosis with cervical spinal cord compression (12), pectus excavatum, thoracic kyphosis and lumbar lordosis (22). Craniofacial/airway morphology is characterized by upper airway stenosis, retruded position of the chin, and increased mandibular plane angle, and increased lower facial height (26). Enlarged tonsils and adenoids play a role in worsening the upper airway obstruction (13). No correlation was found between CA and abnormal magnetic resonance imaging (MRI) suggesting foramen magnum stenosis (27).
Down syndrome
Patients with DS have many predisposing factors for developing OSAS, including midfacial hypoplasia, an abnormally small upper airway with superficially positioned tonsils and relative tonsillar and adenoidal encroachment and obesity (14). OSAS risks was elevated in obese DS children (28,29). The minimal upper airway cross-sectional area, measured with CT scan, was smaller in DS children with severe OSAS (aged 4.3±2.3 years). Children with a less favourable response to A&T had a smaller volume of regions below the tonsils, due to enlargement of the lingual tonsils, glossoptosis, or macroglossia (15).
Prader-Willi syndrome
PWS is a complex genetic condition that affects many parts of the body. In infancy, this condition is characterized by weak muscle tone (hypotonia), delayed development, and distinctive facial features such as a narrow forehead. OSA was the predominant sleep-related disorder in PWS, not associated with age or obesity (30). The weak association with obesity leaded to hypothesize that hypotonia and/or facial dysmorphism play a role in SDB (16).
Ehler-Danlos syndrome
EDS is a group of rare inherited conditions that affect the connective tissue. Several major types are identified including classical, hypermobile, vascular, kyphoscoliotic, arthrochalasic and dermatosparactic. EDS has been suggested as a genetic model for OSA because of abnormalities in oral-facial growth. EDS is characterized by cartilaginous defects, including nasal-maxillary cartilages (17). Abnormal developments of cartilage, including those of the airways, impacts the growth and development of the nose and maxilla (upper jaw) and upper airway stability.
Pierre-Robin sequence/syndrome
PRS is characterized by the triad of micrognathia, glossoptosis, and upper airway obstruction. About 50% of PRS cases are syndromic rather than isolated. The most common syndromes are Stickler syndrome, Velocardiofacial syndrome, and Treacher-Collins syndrome (31). Nasopharyngoscopy have revealed that the etiology of OSA is multifactorial. The upper airway obstruction is caused by anatomical abnormalities, mechanical collapse of the pharyngeal wall, and maxillary hypoplasia (32).
Sickle cell disease
SCD and its variants are genetic disorders resulting from a mutated form of hemoglobin, hemoglobin S (HbS). Children with SCD had a smaller upper airway and larger adenoid and tonsils (20). High prevalences of SDB consistent with OSAS and typical nocturnal symptoms of snoring and breathing/sleep disturbances were reported among 243 children with a median age of 10 years (33). OSAS were associated with higher levels of habitual snoring and lower waking pulse oxygen saturation (SpO2) (33). Through a variety of mechanisms including nocturnal hypoxemia, increased oxidative stress, production of pro-inflammatory cytokines, and endothelial dysfunction, SCD and SDB potentiate each other’s clinical condition and organ complications (34).
Mucopolysaccharidosis
MPS are a group of rare lysosomal storage diseases caused by the deficiency of one of ten specific lysosomal enzymes. Upper airway obstruction is reported in I, II, IV, VI and VII subtypes (35). Supraglottic manifestations are common due to cranial and spinal abnormalities (e.g., flattened nasal bridge, short neck, high epiglottis, mandibular abnormalities) and glycosaminoglycans deposition in the mouth, nose and throat (36-38). Most MPS patients have high airway obstruction from adenotonsillar hypertrophy (39).
Osteogenesis imperfecta
OI, also known as brittle bone disease, is a group of genetic connective tissue disorders that affect the bones (40). The major clinical manifestation of OI is skeletal fragility, but skeletal deformity, joint laxity, and scoliosis may also be present (41). Extraskeletal manifestations include dentinogenesis imperfecta and cranial malformations (Figure 1) such as macrocephaly, hydrocephalus and basilar invagination (42). Malocclusions become more predominant with increasing age. The OI patients have retarded vertical dimensions, a flattened cranial base angle, relative prognathism, larger facial divergence, and more forward counterclockwise mandibular growth (43). Studies showed OSA in OI children and adults secondary to laryngomalacia or redundant supraglottic or epiglottic mucosa (44). A paradoxical inspiratory inward motion of the pulmonary rib cage, thoracoabdominal asynchronies and rib cage distortions characterized the OI type III patients in a supine position (45).
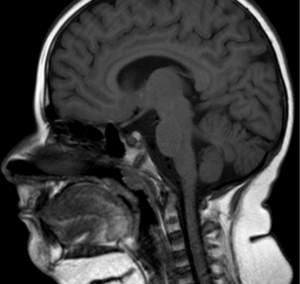
Ellis-van Creveld syndrome
EVC is a chondral and ectodermal dysplasia characterized by short ribs, polydactyly, growth retardation and heart defects. The oral manifestation spectrum is wide (Figure 2), including malocclusion (46). The literature describes hypoplasia of the anterior maxilla, prognathism of the mandible and the increased height of the lower third of the face (19). In EVC children, the prognosis is associated with respiratory difficulties in the first months of life (46).
Noonan syndrome
NS is a genetically transmitted autosomal dominant disorder characterized by distinctive facial deformities, short stature, chest deformity and congenital heart disease (47). The main facial features are hypertelorism with down-slanting palpebral fissures, ptosis, and low-set posteriorly rotated ears with a thickened helix. Additional features are possible, such as a webbed neck and chest deformity (47-49).
Summary remarks
Pathophysiology of SDB in children affected by genetic diseases is addressed but not limited to increased dimensions of linfatic tissues in the neck and facial characteristics predisposing to reduced patency of the high airways. Therefore, in some diseases, muscle hypotonia, connective tissue pathology and morbid fatness take a significant role.
What is the incidence/prevalence of OSAS in genetic syndromes?
Achondroplasia
A review of the medical records of 46 ACH children (mean age, 3.9 years; range, 3 months–14 years) showed that 54% had OSA (22). Sleep investigations were abnormal in 28/30 (93%) of children with ACH (median age 3.0 years, range: 0.4–17.1) of which 37% had an AHI ≥1 event/hr and 87% had AHI ≥5 events/hr (moderate-severe). The desaturation index (ODI ≥3%) was >5 events/hr in 73% of patients (50). A review of the clinical charts of 43 children (mean age 3.9±3.5 years) showed that 59% had OSA (51).
Down syndrome
OSAS is recognized as a consistent problem in DS children. In DS infants ≤6 months of age (n=177) the overall prevalence of OSA was 31% (52). In a review of 50 years of research studies, the prevalence rate of OSAS ranged between 24% and 59% (53). Among 32 of 8-year-old children, 66% had an AHI >5 events/hr and 59% had oAHI >5 events/hr which showed a moderate-severe OSAS (54). Among 44 children (mean age 3.6, min 0.1–max 10 years) OSAS was present in 61% (55). Maris et al. diagnosed OSA in 57.1% of 54 children aged 7.5 (5.4–11.6) years (56). 87% of 23 children, aged 8–19 years, had OSAS (57). Basil et al. found that 74% of 177 children (age range: 2.1–19.1 years) had OSAS and the obese individuals had moderate-severe SDB (28). Among 57 children, with a mean age of 6.2±5.9 years, mean AHI was 14±16 events/hr. 80% patients had OSA with an AHI >1 event/hr and 39% had AHI ≥10 events/hr (severe) (58).
Prader-Willi syndrome
A systematic review of the literature (40 studies) showed a high prevalence of OSA in PWS (79.9%; n=179/224), of which 53.1% had mild OSA, 22.4% moderate OSA, and 24.6% severe OSA (59). OSA was diagnosed in 13/14 patients (92.9%; age range, 8 months–17 years) (30). Central sleep apnea (CSA) was prevalent in infants with PWS but improved with age. Some infants had persistent CSA and others were at risk of developing OSA (60).
Ehler-Danlos syndrome
In EDS adulthood, OSA is a common condition. The affecting prevalence was 32% vs. 6% of healthy controls. OSA severity was associated with daytime sleepiness and lower quality of life (61). OSA is prevalent in 24 school-aged children with EDS (42%) (62). A retrospective review of medical records and PSG tests in 65 children with EDS <18-year-old showed a high prevalence of sleep disorders, including OSA (26%) (63).
Pierre-Robin sequence/syndrome
Infants with PRS commonly have SDB, including OSA and central sleep breathing (CSA). OSA was identified in 11 of 13 (85%) infants (64). Forty-five infants received pre-operative PSG and 80% demonstrated severe sleep apnea (AHI ≥10 events/hr), 69% showed severe OSA (Oahi ≥10 events/hr), and 56% showed CAs (CAI ≥1 event/hr) (65) A retrospective chart review confirms a high prevalence of OSA in infants (aged 0.8±0.3 years). Twenty-two out of 46 (47%) had evidence of OSA (32).
Sickle cell disease
SCD imparts an increased risk for OSA in childhood. The prevalence of OSAS in children with SCD is higher than in the general pediatric population. OSA was diagnosed in 38/55 (69%) children (66). PSG showed that 19.4% (7 of 36) of children with SCD had OSAS (20). It was also present in 41% or 10% children at cut-points of AHI ≥1 or ≥5 events/hr, respectively (33).
Mucopolysaccharidosis
SDB occurs in >80% of MPS patients (35). The incidence of SDB among 61 MPS I (44 Hurler, 17 attenuated) patients (median age of 6.8 years) between 6 months to 16 years post-treatment (following A&T, laryngeal microsurgery or CPAP) was 68%, while 13% (4/30) patients had evidence of severe OSAS (67). Median AHI was 6.4 events/hr in 30 patients with MPS type II (Hunter syndrome) at the median age of 9 years, with OSA observed in 27/30 subjects (68). The prevalence of OSAS in patients with MPS types I, II, and VI was 69.8% (54 patients with MPS subtype I, n=17; II, n=16; and VI; n=12) (69). Forty patients out of 42 tested with PSG (MPS III, IV, VI subtypes) had OSA (95%). There was no significant difference between MPS subtypes according to tonsil grade, adenoid grade, rate of otitis media with effusion and OSAS severity (39).
Osteogenesis imperfecta
In adulthood with OI, sleep disturbances appeared to be common, but remain frequently undiagnosed (70). Mild sleep respiratory disturbance was reported in most cases of OI, while in a minority, were observed significant desaturations during sleep (71). Clinical charts of 188 patients referred to genetic skeletal disorders reference center for OI showed that among the 15 patients (8%) that performed a PSG, 12 patients (6.4%) had SDB (72).
Ellis-van Creveld syndrome
Data from the literature are missing regarding SDB in these patients. Mild respiratory disturbance in one (5.6-year-old) out of two EVC patients, due to hypopnea, was observed with contextually an increased ODI.
Noonan syndrome
Data on incidence and prevalence of OSAS (Figure 3) in NS are unavailable.
Summary remarks
A body of the literature showed that OSAS is well recognized in children with ACH, DS, PWS, EDS, PRS and MPS suggesting that it is a dominant characteristic in these syndromes. In SCD the prevalence of OSAS is recognized high and requires clinical consideration in its management. In children with EVC syndrome, NS and OI, data in the literature are insufficient.
What result from management and prognosis in genetic syndromes?
Achondroplasia
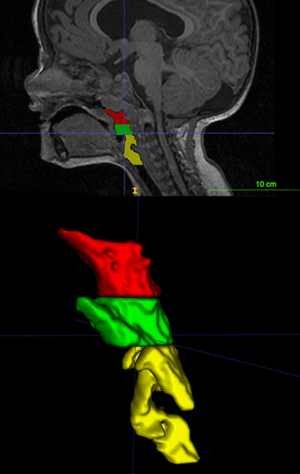
Adenotonsillectomy (A&T) was effective in improve sleep respiratory disturbances in the long term (73). Children who underwent A&T, coupled with turbinectomy, were older (mean age 7.5±3.5 years old) and had improved PSG results than those who underwent only adeno-turbinectomy (3.5±1.7 years old, P=0.015) (51). The persistence of significant OSA after A&T is due to the reduced base of the skull and hypoplasia of the middle third of the face (50). Sleep respiratory disturbances correlated negatively with the relative nasopharynx plus oropharynx space measured at MRI (Figure 4). The smaller the relative nasopharynx plus oropharynx space, the higher the number of OAs during sleep. It was recently observed that starting orthodontic treatment (RME), as soon as OSAS symptoms appear, may be a valuable approach that increased treatment efficacy (74). ACH children with OSAS, who do not benefit from A&T, should be treated with nasal CPAP therapy (75).
Down syndrome
DS children are more susceptible to the additional negative impact of sleep respiratory disturbance since they have frequent pre-existing medical and neurocognitive disabilities. OSAS has been associated with cardiovascular complication (i.e., pulmonary hypertension) (14). Monotherapy was insufficient (76). In a cohort study including 41 males and 34 females (age of 5.1±3.6 years), tonsillectomy resulted in significant improvements in multiple sleep respiratory parameters (77). Tonsillectomy resolved 30–50% of OSA (53). Thirty-four children (median age, 4.0 years; range, 2.7–5.8 years), showed a significant improvement of AHI from 11.4 (range, 6.5–22.7) events/hr to 3.6 (range, 2.1–9.5) events/hr after A&T, with a parallel increase of the minimum SpO2 (78). Therefore, after A&T, 69% (of 33 children; aged 4.3±2.3 years) had persistent OSA (oAHI >2 events/hr). A greater than 50% decrease in oAHI was observed in 79% of patients, associated to a higher air volume of the regions below the tonsils (15).
Prader-Willi syndrome
A systematic review showed that A&T was effective in reducing OSA for some PWS children, but alternative treatments may be considered, given the only moderate response rate (59). OAHI decreased after A&T in 22 children, but a significant number had persistent OSA (79). Velopharyngeal dysfunctions (VPD) may occur after A&T (80). Oxygen therapy resulted in a significant decrease in the median CAI (81).
Ehler-Danlos syndrome
Recommendation for EDS has lacked so far. Management is helpful for all diseases involving connective tissue (82).
Pierre-Robin sequence/syndrome
Children with PRS, who needed respiratory support early after birth, were at risk of continuing or re-developing OSA after the age of 1 year. Between the age of 1 and 18 years, almost one out of four children with PRS had respiratory problems (83). International guidelines recommended the surgical management of children with PRS who failed conservative therapy (65). Among 9 patients who underwent mandibular distraction osteogenesis, with pre- and post-operative PSGs, significant reductions in AHI and CAI was reported (65). Mandibular distraction osteogenesis was the most common primary procedure, followed by tongue-lip adhesion, and tracheostomy (84). A meta-analysis of 7 studies with 90 patients showed that tongue-lip adhesion and tongue repositioning can improve AHI and oxygenation parameters during sleep (85).
Sickle cell disease
In children (n=256) with SCD, A&T was associated with a reduced rate of visits over time for OSA and of cerebrovascular ischemia (e.g., stroke, transient ischemic attacks) events (86). In 13 children with SCD, a significant reduction in hemoglobin oxygen desaturation, decreased AHI, occurred after A&T (87). Among 15 children (aged from 2 to 18 years) with a history of SCD and OSA followed by A&T there was a significant reduction in mean (95% CI) cerebral blood flow velocities (88).
Mucopolysaccharidosis
MPS patients have airway obstruction and OSA due to adenotonsillar hypertrophy. MPS have a high prevalence of OSAS complicated by pulmonary hypertension (8). Ear, nose and throat surgery reduced the frequency and relieved the symptoms related to upper airway obstruction (89). Most of these children advantaged from A&T (35,39). Lingual tonsils hypertrophy could cause persistent OSA in children after A&T (90). A&T and enzyme replacement therapy may decrease OSAS severity (8). Interventions maximizing substrate reduction (enzyme replacement therapy) correlated with long-term SDB improvement (67). Hematopoietic stem cell transplantation did not offer long-term protection against OSAS in MPS type I (91).
Osteogenesis imperfecta
Literature data on management and prognosis in OI are insufficient. Few OI patients were started on CPAP, with clinical improvement (72).
Ellis-van Creveld syndrome
Data on management and prognosis in EVC syndrome are insufficient. Among OSA patients (n=17; 26%), two required CPAP (63).
Noonan syndrome
Data on management and prognosis in NS are insufficient. Khirani et al. (92) reported a 15-month-old boy with severe OSAS and moderate hypertrophic cardiomyopathy. After adenoidectomy, PSG confirmed the recovery (92). In our case, clinical improvement after adenoidectomy was also great, but the problem was not completely resolved at follow-up. In many children with craniofacial disorders, soft tissue correction by adenoidectomy did not always remedy the airway obstruction (93).
Summary remarks
A&T was advised in children with ACH, DS, PWS, SCD and MPS with OSAS, although most of them did not resolve the problem, requiring further interventions. Children with PRS required surgical management. Data on EDS, EVC syndrome, NS and OI do not make comprehensive suggestions.
What are the recommendations in genetic syndromes?
Achondroplasia
A&T is like an important treatment option in ACH children with OSAS (94). Early detections of sleep disorders are recommended to these children (95), including the use of PSG and imaging (96). Before performing A&T, the clinician should refer children with craniofacial abnormalities for PSG (11).
Down syndrome
An important role of PSG has been advocated in characterizing breathing abnormalities in children with DS (10). Formal screening tools for OSA is addressed to improve detection of this high-risk patients (97). The American Academy of Pediatrics recommended a referrals to a pediatric sleep laboratory for all children with DS by 4 years of age (98). Before performing tonsillectomy, the clinician should refer for PSG all DS children with SDB (99). In children with DS, PSG screening is mandatory, considering the potential overall morbidities of untreated OSAS (55).
Prader-Willi syndrome
Children with PWS being or not considered for growth hormone (GH) replacement therapy should be assessed for OSA by PSG (10,59,100).
Pierre-Robin sequence/syndrome
Before performing tonsillectomy, the clinician should refer children with SDB for PSG if they exhibit craniofacial abnormalities (11). Significant OSAS is often present in infants with PRS, and PSG is useful in testing sleep breathing disorders (10). PRS should be screened for OSA because the incidence is high, and signs may be subtle. Symptoms indicative of OSA such as snoring are not always present (84). PRS infants often require early and long-term upper airway management (65). Those who needed respiratory support at an early age looked for careful monitoring until adulthood (83).
Sickle cell disease
OSAS in children with SCD was associated with high rates of a broad range of complications, including pneumonia and acute chest syndrome. Routine screenings, diagnosis, and increased therapeutic intervention for children with comorbid OSA decreased SCD morbidity (101). Before performing tonsillectomy, the clinician should refer SCD children for PSG screening (11).
Mucopolysaccharidosis
In children with MPS, before performing tonsillectomy, the clinician should discuss children for PSG (11). PSG should be offered in all patients after diagnosis of MPS (8,35).
Osteogenesis imperfecta
OSAS in OI children should be systematically searched (72).
Summary remarks
Recommendations are well established in children with ACH, DS, SCD, MPS and in general in those with craniofacial abnormalities (PRS). In children with EDS, EVC, NS and OI are not stated.
Discussion and conclusions
American Academy of Pediatrics (AAP) published in 2002 the clinical practice guideline of diagnosis and management of childhood OSAS (102). In this statement, complex high-risk patients (i.e., DS, SCD, genetic/metabolic/storage diseases, craniofacial disorders) should be referred to a sleep specialist. Before performing A&T, other authors recommended for PSG all children, particularly if had DS, SCD, MPS and craniofacial abnormalities (10,11,103). Children with DS have multiple anatomic and physiologic conditions that predispose to OSAS (hypotonia, macroglossia) (104). Nocturnal hypoxemia is common in SCD, because of upper airway obstruction secondary to adenotonsillar hypertrophy (105). SDB are common to children with MPS because of upper airway narrowing caused by hypertrophy of the tongue, tonsils, adenoids, and mucous membranes (106). Children with craniofacial syndromes are at a high risk of SDB because of oropharyngeal and nasopharyngeal crowding and laryngeal abnormalities (107). Interestingly, revised clinical practice guideline in 2012 included but not limited to DS, SCD, metabolic disease and craniofacial anomalies, the investigation and management of OSAS (108).
Patients with a complex medical condition are by the time established to be at high-risk for OSAS (i.e., ACH, DS, PWS, SCD, MPS) including also the condition so-called neuromuscular disorders, and genetic syndromes with craniofacial anomalies. These complex patients with OSA would require a sleep study first, followed by an implementation of an action plan (109). The complexity of these patients is clarified because isolated A&T can rarely cure OSAS, causing multidisciplinary approaches (110). Thus, other genetic pathologies, as neuromuscular disorders (i.e., Duchenne muscular dystrophy) or syndromes with craniofacial dysostosis (Apert, Crouzon, and Pfeiffer syndromes) or other form of micrognathia (Treacher Collins syndrome, Nager syndrome) fallen under this definition (10,104), for which were addressed general recommendations for their management (103).
It has been argued an “intentional vagueness” concerning the use of a broad category of neuromuscular disorders and craniofacial anomalies rather than a comprehensive list of diseases and syndromes, to emphasize the need for individualized management (11). Several genetic diseases are inconclusive regarding the association with sleep breathing pathology, and for some other conditions, the published studies regarded only case reports.
Recently, the European Respiratory Society (ERS) task force, in line with the American Academy of Otolaryngology-Head and Neck Surgery, recommended that children with co-existing DS, SCD, MPS, neuromuscular disorders or craniofacial abnormalities, or children in whom the need for treatment is unclear, should have the priority in accessing PSG before A&T. These children should also have a PSG post A&T due to their increased risk of persistent OSA (8). Studies have showed a high prevalence of OSAS in children with ACH (midface hypoplasia and brainstem compression). Children with DS are predisposed to OSAS and hypoventilation while parents may not declare SDB symptoms. EDS has been associated with SDB. MPS represent a group of rare lysosomal storage diseases and the reported high prevalence of OSAS has been attributed to the narrowed upper airway lumen. Most children with PWS have OSAS, mostly of mild severity (8,10). These characteristics highlights the absolute need for systematic exploration, to avoid unwarily in attributing an intellectual or neurocognitive deficit to the main pathology (110).
A high prevalence of sleep disturbances is explained for some genetic conditions (Table 2). OSAS takes a fundamental part of the disease characteristics in ACH, DS, PWS and PRS and should not be considered an incidental complication. Among groups of genetic diseases, MPS is established for OSAS complication. They have infiltration and accumulation of macromolecules in the tissues around the upper airway. OSAS, compensatory lymphoid hyperplasia secondary to functional hyposplenism and defects in immune surveillance are described in SCD. Reactive lymphoid hyperplasia may be from repeated infections. Upper airway obstruction during sleep due to adenotonsillar enlargement has been found in up to one-third of children with SCD. Thus, MPS and SCD are at high risk for OSAS and should be considered in the overall management of these patients. Recent findings claimed a high prevalence of sleep disorders in EDS with excessive daytime sleepiness and impaired quality of life (61,63). Insufficient evidence is available regarding the role of SDB in children with EVC and NS. In OI patients the data are also insufficient but relevant if associated with high BMI, trunk deformations, and in the severe OI subtypes (72). Finally, data are insufficient in EDS and OI to make comprehensive conclusions.

Full table
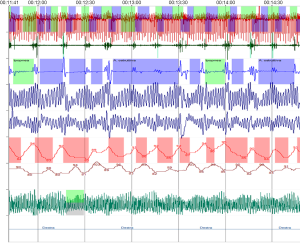
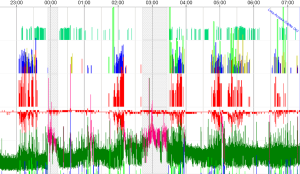
Several other genetic conditions suffered from OSAS. For example, Follmar et al. reported a prevalence of SDB in the 118 patients with Beckwith-Wiedemann syndrome (BWS) was 48% (n=57). The aetiology of SDB in these patients is multifactorial but may not be solely the result of a large tongue (111). Recently, we tested a female child with BWS (7-year-old) before spinal intervention for severe scoliosis, showing clusters of desaturations, severe AHI (25.1 events/hr) and ODI (13.9 events/hr) (Figure 5). The measure of daytime and nocturnal etCO2 was in range. The data on SDB in these patients are closed to recent findings.
Looking for guidelines with a comprehensive list of genetic diseases and syndromes at high-risk for OSAS, clinicians, pediatric geneticists and pediatric metabolic specialists, must be alert to signs and symptoms of SDB or in children affected by anatomic conditions that can increase the risk of SDB. Since symptoms of OSAS are not frequently overt, all children with genetic syndromes affecting connective tissue, craniofacial malformations, storage diseases, morbid obesity should be beheld for SDB screening. PSG study, included in clinical practice, is helpful to screen for suspected for OSAS in all children with genetic diseases.
The advantage of a widespread screening and—in the case—of the treatment of OSAS in children with genetic diseases is reasonable. The associated complications of OSAS are inattention and behavioural problems, daytime sleepiness, failure to thrive, cardiological and metabolic involvement. The goals of the further efforts can be the inclusion of various genetic diseases into guidelines for the screening of OSAS, updating the shreds of evidence based on the research progression.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wilhelm CP, deShazo RD, Tamanna S, et al. The nose, upper airway, and obstructive sleep apnea. Ann Allergy Asthma Immunol 2015;115:96-102. [Crossref] [PubMed]
- White DP. The pathogenesis of obstructive sleep apnea: advances in the past 100 years. Am J Respir Cell Mol Biol 2006;34:1-6. [Crossref] [PubMed]
- Tawfik KO, Sedaghat AR, Ishman SL. Trends in Inpatient Pediatric Polysomnography for Laryngomalacia and Craniofacial Anomalies. Ann Otol Rhinol Laryngol 2016;125:82-9. [Crossref] [PubMed]
- Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:242-52. [Crossref] [PubMed]
- Zaffanello M, Piacentini G, Gasperi E, et al. Snoring in a cohort of obese children: Association with palate position and nocturnal desaturations. JPNIM 2016;5.
- Zaffanello M, Piacentini G, Pietrobelli A, et al. Ambulatory clinical parameters and sleep respiratory events in a group of obese children unselected for respiratory problems. World J Pediatr 2017;13:577-83. [Crossref] [PubMed]
- Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130:e714-55. [Crossref] [PubMed]
- Kaditis AG, Alonso Alvarez ML, Boudewyns A, et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J 2016;47:69-94. [Crossref] [PubMed]
- Boudewyns A, Abel F, Alexopoulos E, et al. Adenotonsillectomy to treat obstructive sleep apnea: Is it enough? Pediatr Pulmonol 2017;52:699-709. [Crossref] [PubMed]
- Wise MS, Nichols CD, Grigg-Damberger MM, et al. Executive summary of respiratory indications for polysomnography in children: an evidence-based review. Sleep 2011;34:389-98AW.
- Roland PS, Rosenfeld RM, Brooks LJ, et al. Clinical practice guideline: Polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg 2011;145:S1-15. [Crossref] [PubMed]
- Onodera K, Sakata H, Niikuni N, et al. Survey of the present status of sleep-disordered breathing in children with achondroplasia Part I. A questionnaire survey. Int J Pediatr Otorhinolaryngol 2005;69:457-61. [Crossref] [PubMed]
- Collins WO, Choi SS. Otolaryngologic manifestations of achondroplasia. Arch Otolaryngol Head Neck Surg 2007;133:237-44. [Crossref] [PubMed]
- Cielo CM, Konstantinopoulou S, Hoque R. OSAS in Specific Pediatric Populations. Curr Probl Pediatr Adolesc Health Care 2016;46:11-8. [Crossref] [PubMed]
- Slaats MA, Loterman D, van Holsbeke C, et al. The Role of Functional Respiratory Imaging in Treatment Selection of Children With Obstructive Sleep Apnea and Down Syndrome. J Clin Sleep Med 2018;14:651-9. [Crossref] [PubMed]
- Pavone M, Caldarelli V, Khirani S, et al. Sleep disordered breathing in patients with Prader-Willi syndrome: A multicenter study. Pediatr Pulmonol 2015;50:1354-9. [Crossref] [PubMed]
- Guilleminault C, Primeau M, Chiu HY, et al. Sleep-disordered breathing in Ehlers-Danlos syndrome: a genetic model of OSA. Chest 2013;144:1503-11. [Crossref] [PubMed]
- Robin P. Glossoptosis due to atresia and hypotrophy of the mandible. American Journal of Diseases of Children 1934;48:541-7.
- Kalaskar R, Kalaskar AR. Oral manifestations of Ellis-van Creveld syndrome. Contemp Clin Dent 2012;3:S55-9. [Crossref] [PubMed]
- Strauss T, Sin S, Marcus CL, et al. Upper airway lymphoid tissue size in children with sickle cell disease. Chest 2012;142:94-100. [Crossref] [PubMed]
- Cardiel Ríos SA. Correction of a severe Class II malocclusion in a patient with Noonan syndrome. American Journal of Orthodontics and Dentofacial Orthopedics 2016;150:511-20. [Crossref] [PubMed]
- Afsharpaiman S, Sillence DO, Sheikhvatan M, et al. Respiratory events and obstructive sleep apnea in children with achondroplasia: investigation and treatment outcomes. Sleep Breath 2011;15:755-61. [Crossref] [PubMed]
- Zaffanello M, Lo Tartaro P, Piacentini G, et al. Sleep disordered breathing in a cohort of children with achondroplasia: correlation between clinical and instrumental findings. Minerva Pediatr 2017;69:481-8. [PubMed]
- Zaffanello M, Cantalupo G, Piacentini G, et al. Sleep disordered breathing in children with achondroplasia. World J Pediatr 2017;13:8-14. [Crossref] [PubMed]
- Zaffanello M, Piacentini G, Sacchetto L, et al. Sleep-Disordered Breathing in Children with Rare Skeletal Disorders: A Survey of Clinical Records. Med Princ Pract 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Onodera K, Niikuni N, Chigono T, et al. Sleep disordered breathing in children with achondroplasia. Part 2. Relationship with craniofacial and airway morphology. Int J Pediatr Otorhinolaryngol 2006;70:453-61. [Crossref] [PubMed]
- White KK, Parnell SE, Kifle Y, et al. Is there a correlation between sleep disordered breathing and foramen magnum stenosis in children with achondroplasia? Am J Med Genet A 2016;170A:32-41. [Crossref] [PubMed]
- Basil JS, Santoro SL, Martin LJ, et al. Retrospective Study of Obesity in Children with Down Syndrome. J Pediatr 2016;173:143-8. [Crossref] [PubMed]
- Shires CB, Anold SL, Schoumacher RA, et al. Body mass index as an indicator of obstructive sleep apnea in pediatric Down syndrome. Int J Pediatr Otorhinolaryngol 2010;74:768-72. [Crossref] [PubMed]
- Canora A, Franzese A, Mozzillo E, et al. Severe obstructive sleep disorders in Prader-Willi syndrome patients in southern Italy. Eur J Pediatr 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Giudice A, Barone S, Belhous K, et al. Pierre Robin Sequence: A comprehensive narrative review of the literature over time. J Stomatol Oral Maxillofac Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Khayat A, Bin-Hassan S, Al-Saleh S. Polysomnographic findings in infants with Pierre Robin sequence. Ann Thorac Med 2017;12:25-9. [Crossref] [PubMed]
- Rosen CL, Debaun MR, Strunk RC, et al. Obstructive sleep apnea and sickle cell anemia. Pediatrics 2014;134:273-81. [Crossref] [PubMed]
- Raghunathan VM, Whitesell PL, Lim SH. Sleep-disordered breathing in patients with sickle cell disease. Ann Hematol 2018;97:755-62. [PubMed]
- Berger KI, Fagondes SC, Giugliani R, et al. Respiratory and sleep disorders in mucopolysaccharidosis. Journal of Inherited Metabolic Disease 2013;36:201-10. [Crossref] [PubMed]
- John A, Fagondes S, Schwartz I, et al. Sleep abnormalities in untreated patients with mucopolysaccharidosis type VI. Am J Med Genet A 2011;155A:1546-51. [Crossref] [PubMed]
- Lin HY, Chen MR, Lin CC, et al. Polysomnographic characteristics in patients with mucopolysaccharidoses. Pediatr Pulmonol 2010;45:1205-12. [Crossref] [PubMed]
- Keilmann A, Läßig AK, Pollak-Hainz A, et al. Adenoids of patients with mucopolysaccharidoses demonstrate typical alterations. International Journal of Pediatric Otorhinolaryngology 2015;79:115-8. [Crossref] [PubMed]
- Gönüldaş B, Yilmaz T, Sivri HS, et al. Mucopolysaccharidosis: Otolaryngologic findings, obstructive sleep apnea and accumulation of glucosaminoglycans in lymphatic tissue of the upper airway. Int J Pediatr Otorhinolaryngol 2014;78:944-9. [Crossref] [PubMed]
- Martin E, Shapiro JR. Osteogenesis imperfecta:epidemiology and pathophysiology. Curr Osteoporos Rep 2007;5:91-7. [Crossref] [PubMed]
- Arponen H, Makitie O, Waltimo-Siren J. Association between joint hypermobility, scoliosis, and cranial base anomalies in paediatric Osteogenesis imperfecta patients: a retrospective cross-sectional study. BMC Musculoskelet Disord 2014;15:428. [Crossref] [PubMed]
- Lamanna A, Fayers T, Clarke S, et al. Valvular and aortic diseases in osteogenesis imperfecta. Heart Lung Circ 2013;22:801-10. [Crossref] [PubMed]
- Chang PC, Lin SY, Hsu KH. The craniofacial characteristics of osteogenesis imperfecta patients. Eur J Orthod 2007;29:232-7. [Crossref] [PubMed]
- Li HY, Fang TJ, Lin JL, et al. Laryngomalacia causing sleep apnea in an osteogenesis imperfecta patient. Am J Otolaryngol 2002;23:378-81. [Crossref] [PubMed]
- LoMauro A, Pochintesta S, Romei M, et al. Rib cage deformities alter respiratory muscle action and chest wall function in patients with severe osteogenesis imperfecta. PLoS One 2012;7. [Crossref] [PubMed]
- Baujat G, Le Merrer M. Ellis-van Creveld syndrome. Orphanet J Rare Dis 2007;2:27. [Crossref] [PubMed]
- Romano AA, Allanson JE, Dahlgren J, et al. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics 2010;126:746-59. [Crossref] [PubMed]
- Kruszka P, Porras AR, Addissie YA, et al. Noonan syndrome in diverse populations. Am J Med Genet A 2017;173:2323-34. [Crossref] [PubMed]
- Roberts AE, Allanson JE, Tartaglia M, et al. Noonan syndrome. Lancet 2013;381:333-42. [Crossref] [PubMed]
- Julliand S, Boule M, Baujat G, et al. Lung function, diagnosis, and treatment of sleep-disordered breathing in children with achondroplasia. Am J Med Genet A 2012;158A:1987-93. [Crossref] [PubMed]
- Tenconi R, Khirani S, Amaddeo A, et al. Sleep-disordered breathing and its management in children with achondroplasia. Am J Med Genet A 2017;173:868-78. [Crossref] [PubMed]
- Goffinski A, Stanley MA, Shepherd N, et al. Obstructive sleep apnea in young infants with Down syndrome evaluated in a Down syndrome specialty clinic. Am J Med Genet A 2015;167A:324-30. [Crossref] [PubMed]
- Churchill SS, Kieckhefer GM, Landis CA, et al. Sleep measurement and monitoring in children with Down syndrome: a review of the literature, 1960-2010. Sleep Med Rev 2012;16:477-88. [Crossref] [PubMed]
- Austeng ME, Overland B, Kvaerner KJ, et al. Obstructive sleep apnea in younger school children with Down syndrome. Int J Pediatr Otorhinolaryngol 2014;78:1026-9. [Crossref] [PubMed]
- Brockmann PE, Damiani F, Nuñez F, et al. Sleep-disordered breathing in children with Down syndrome: Usefulness of home polysomnography. Int J Pediatr Otorhinolaryngol 2016;83:47-50. [Crossref] [PubMed]
- Maris M, Verhulst S, Wojciechowski M, et al. Sleep problems and obstructive sleep apnea in children with down syndrome, an overwiew. Int J Pediatr Otorhinolaryngol 2016;82:12-5. [Crossref] [PubMed]
- Konstantinopoulou S, Tapia IE, Kim JY, et al. Relationship between obstructive sleep apnea cardiac complications and sleepiness in children with Down syndrome. Sleep Med 2016;17:18-24. [Crossref] [PubMed]
- Dudoignon B, Amaddeo A, Frapin A, et al. Obstructive sleep apnea in Down syndrome: Benefits of surgery and noninvasive respiratory support. Am J Med Genet A 2017;173:2074-80. [Crossref] [PubMed]
- Sedky K, Bennett DS, Pumariega A. Prader Willi syndrome and obstructive sleep apnea: co-occurrence in the pediatric population. J Clin Sleep Med 2014;10:403-9. [PubMed]
- Khayat A, Narang I, Bin-Hasan S, et al. Longitudinal evaluation of sleep disordered breathing in infants with Prader-Willi syndrome. Arch Dis Child 2017;102:634-8. [Crossref] [PubMed]
- Gaisl T, Giunta C, Bratton DJ, et al. Obstructive sleep apnoea and quality of life in Ehlers-Danlos syndrome: a parallel cohort study. Thorax 2017;72:729-35. [Crossref] [PubMed]
- Stöberl A, Gaisl T, Sievi N, et al. Obstructive sleep apnea in children and adolescents with Ehlers-Danlos syndrome. Eur Respir J 2017;50.
- Domany KA, Hantragool S, Smith DF, et al. Sleep Disorders and Their Management in Children With Ehlers-Danlos Syndrome Referred to Sleep Clinics. J Clin Sleep Med 2018;14:623-9. [Crossref] [PubMed]
- Anderson IC, Sedaghat AR, McGinley BM, et al. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence. Cleft Palate Craniofac J 2011;48:614-8. [Crossref] [PubMed]
- Lee JJ, Thottam PJ, Ford MD, et al. Characteristics of sleep apnea in infants with Pierre-Robin sequence: Is there improvement with advancing age? Int J Pediatr Otorhinolaryngol 2015;79:2059-67. [Crossref] [PubMed]
- Rogers VE, Lewin DS, Winnie GB, et al. Polysomnographic characteristics of a referred sample of children with sickle cell disease. J Clin Sleep Med 2010;6:374-81. [PubMed]
- Pal AR, Langereis EJ, Saif MA, et al. Sleep disordered breathing in mucopolysaccharidosis I: a multivariate analysis of patient, therapeutic and metabolic correlators modifying long term clinical outcome. Orphanet J Rare Dis 2015;10:42. [Crossref] [PubMed]
- Wooten WI 3rd, Muenzer J, Vaughn BV, et al. Relationship of sleep to pulmonary function in mucopolysaccharidosis II. J Pediatr 2013;162:1210-5. [Crossref] [PubMed]
- Moreira GA, Kyosen SO, Patti CL, et al. Prevalence of obstructive sleep apnea in patients with mucopolysaccharidosis types I, II, and VI in a reference center. Sleep Breath 2014;18:791-7. [Crossref] [PubMed]
- Arponen H, Waltimo-Siren J, Valta H, et al. Fatigue and disturbances of sleep in patients with osteogenesis imperfecta - a cross-sectional questionnaire study. BMC Musculoskelet Disord 2018;19:3. [Crossref] [PubMed]
- Ross KR, Rosen CL. Sleep and respiratory physiology in children. Clin Chest Med 2014;35:457-67. [Crossref] [PubMed]
- Léotard A, Taytard J, Aouate M, et al. Diagnosis, follow-up and management of sleep-disordered breathing in children with osteogenesis imperfecta. Ann Phys Rehabil Med 2018;61:135-9. [Crossref] [PubMed]
- Patino M, Sadhasivam S, Mahmoud M. Obstructive sleep apnoea in children: perioperative considerations. Br J Anaesth 2013;111 Suppl 1:i83-95. [Crossref] [PubMed]
- Villa MP, Rizzoli A, Rabasco J, et al. Rapid maxillary expansion outcomes in treatment of obstructive sleep apnea in children. Sleep Med 2015;16:709-16. [Crossref] [PubMed]
- Schlüter B, De Sousa G, Trowitzsch E, et al. Diagnostics and management of sleep-related respiratory disturbances in children with skeletal dysplasia caused by FGFR3 mutations (achondroplasia and hypochondroplasia). Georgian Med News 2011.63-72. [PubMed]
- Farhood Z, Isley JW, Ong AA, et al. Adenotonsillectomy outcomes in patients with Down syndrome and obstructive sleep apnea. Laryngoscope 2017;127:1465-70. [Crossref] [PubMed]
- Ingram DG, Ruiz AG, Gao D, et al. Success of Tonsillectomy for Obstructive Sleep Apnea in Children With Down Syndrome. J Clin Sleep Med 2017;13:975-80. [Crossref] [PubMed]
- Maris M, Verhulst S, Wojciechowski M, et al. Outcome of adenotonsillectomy in children with Down syndrome and obstructive sleep apnoea. Arch Dis Child 2017;102:331-6. [Crossref] [PubMed]
- Padia R, Muntz H, Pfeffer K, et al. Effectiveness of Adenotonsillectomy and Risk of Velopharyngeal Insufficiency in Children With Prader-Willi Syndrome. Ann Otol Rhinol Laryngol 2017;126:733-8. [Crossref] [PubMed]
- Crockett DJ, Ahmed SR, Sowder DR, et al. Velopharyngeal dysfunction in children with Prader-Willi syndrome after adenotonsillectomy. Int J Pediatr Otorhinolaryngol 2014;78:1731-4. [Crossref] [PubMed]
- Cohen M, Hamilton J, Narang I. Clinically important age-related differences in sleep related disordered breathing in infants and children with Prader-Willi Syndrome. PLoS One 2014;9. [Crossref] [PubMed]
- Esteller E. Obstructive sleep apnea-hypopnea syndrome in children: beyond adenotonsillar hypertrophy. Acta Otorrinolaringol Esp 2015;66:111-9. [Crossref] [PubMed]
- van Lieshout MJS, Joosten KFM, Koudstaal MJ, et al. Management and outcomes of obstructive sleep apnea in children with Robin sequence, a cross-sectional study. Clin Oral Investig 2017;21:1971-8. [Crossref] [PubMed]
- Tan HL, Kheirandish-Gozal L, Abel F, et al. Craniofacial syndromes and sleep-related breathing disorders. Sleep Med Rev 2016;27:74-88. [Crossref] [PubMed]
- Camacho M, Noller MW, Zaghi S, et al. Tongue-lip adhesion and tongue repositioning for obstructive sleep apnoea in Pierre Robin sequence: A systematic review and meta-analysis. J Laryngol Otol 2017;131:378-83. [Crossref] [PubMed]
- Tripathi A, Jerrell JM, Stallworth JR. Cost-effectiveness of adenotonsillectomy in reducing obstructive sleep apnea, cerebrovascular ischemia, vaso-occlusive pain, and ACS episodes in pediatric sickle cell disease. Ann Hematol 2011;90:145-50. [Crossref] [PubMed]
- Finch P, Stocks RM, Smeltzer MP, et al. Effects of adenotonsillectomy on polysomnographic parameters in children with sickle cell disease. Pediatr Blood Cancer 2013;60:E26-8. [Crossref] [PubMed]
- Santarelli G, DeShields SC, Ishman SL, et al. Changes in Transcranial Ultrasound Velocities in Children with Sickle Cell Disease Undergoing Adenotonsillectomy. Otolaryngol Head Neck Surg 2018;158:942-6. [Crossref] [PubMed]
- Mesolella M, Cimmino M, Cantone E, et al. Management of otolaryngological manifestations in mucopolysaccharidoses: our experience. Acta Otorhinolaryngol Ital 2013;33:267-72. [PubMed]
- Abdel-Aziz M, Ibrahim N, Ahmed A, et al. Lingual tonsils hypertrophy; a cause of obstructive sleep apnea in children after adenotonsillectomy: operative problems and management. Int J Pediatr Otorhinolaryngol 2011;75:1127-31. [Crossref] [PubMed]
- Moreau J, Brassier A, Amaddeo A, et al. Obstructive sleep apnea syndrome after hematopoietic stem cell transplantation in children with mucopolysaccharidosis type I. Mol Genet Metab 2015;116:275-80. [Crossref] [PubMed]
- Khirani S, Leboulanger N, Ramirez A, et al. Life-threatening obstructive sleep apnea caused by adenoid hypertrophy in an infant with noonan syndrome. Case Rep Pediatr 2012;2012. [Crossref] [PubMed]
- Garg RK, Afifi AM, Garland CB, et al. Pediatric Obstructive Sleep Apnea: Consensus, Controversy, and Craniofacial Considerations. Plast Reconstr Surg 2017;140:987-97. [Crossref] [PubMed]
- Waters KA, Everett F, Sillence DO, et al. Treatment of obstructive sleep apnea in achondroplasia: evaluation of sleep, breathing, and somatosensory-evoked potentials. Am J Med Genet 1995;59:460-6. [Crossref] [PubMed]
- Trotter TL, Hall JG. American Academy of Pediatrics Committee on G. Health supervision for children with achondroplasia. Pediatrics 2005;116:771-83. [Crossref] [PubMed]
- White KK, Bompadre V, Goldberg MJ, et al. Best practices in the evaluation and treatment of foramen magnum stenosis in achondroplasia during infancy. Am J Med Genet A 2016;170A:42-51. [Crossref] [PubMed]
- Lin SC, Davey MJ, Horne RS, et al. Screening for obstructive sleep apnea in children with Down syndrome. J Pediatr 2014;165:117-22. [Crossref] [PubMed]
- Bull MJ. Genetics Co. Health supervision for children with Down syndrome. Pediatrics 2011;128:393-406. [Crossref] [PubMed]
- Aurora RN, Zak RS, Karippot A, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep 2011;34:379-88. [Crossref] [PubMed]
- DeMarcantonio MA, Darrow DH, Gyuricsko E, et al. Obstructive sleep disorders in Prader-Willi syndrome: The role of surgery and growth hormone. Int J Pediatr Otorhinolaryngol 2010;74:1270-2. [Crossref] [PubMed]
- Katz T, Schatz J, Roberts CW. Comorbid obstructive sleep apnea and increased risk for sickle cell disease morbidity. Sleep Breath 2018;22:797-804. [Crossref] [PubMed]
- Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:704-12. [Crossref] [PubMed]
- Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J 2011;37:1000-28. [Crossref] [PubMed]
- Levanon A, Tarasiuk A, Tal A. Sleep characteristics in children with Down syndrome. J Pediatr 1999;134:755-60. [Crossref] [PubMed]
- Wali YA, al-Lamki Z, Soliman H, et al. Adenotonsillar hypertrophy: a precipitating factor of cerebrovascular accident in a child with sickle cell anemia. J Trop Pediatr 2000;46:246-8. [Crossref] [PubMed]
- Leighton SE, Papsin B, Vellodi A, et al. Disordered breathing during sleep in patients with mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol 2001;58:127-38. [Crossref] [PubMed]
- Nishikawa H, Pearman K, Dover S. Multidisciplinary management of children with craniofacial syndromes with particular reference to the airway. Int J Pediatr Otorhinolaryngol 2003;67 Suppl 1:S91-3. [Crossref] [PubMed]
- Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130:576-84. [Crossref] [PubMed]
- Kothare SV, Rosen CL, Lloyd RM, et al. Quality measures for the care of pediatric patients with obstructive sleep apnea. J Clin Sleep Med 2015;11:385-404. [PubMed]
- Fauroux B, Amaddeo A. The particular case of sleep-disordered breathing in syndromic patients. J Dentofacial Anom Orthod 2015;18:305. [Crossref]
- Follmar A, Dentino K, Abramowicz S, et al. Prevalence of sleep-disordered breathing in patients with Beckwith-Wiedemann syndrome. J Craniofac Surg 2014;25:1814-7. [Crossref] [PubMed]


