Overcoming the limitations of glomerular filtration rate estimation by using a novel rapid bedside measurement?
Introduction
Kidney function cannot be measured directly. Glomerular filtration rate (GFR) is the most widely used surrogate marker for drug dosing and monitoring progression of chronic kidney disease (CKD) (1,2). The gold standard is inulin clearance (2,3). Inulin is a natural storage carbohydrate present in more than 36,000 species of plants, including wheat, onion, bananas, garlic, asparagus, Jerusalem artichoke and chicory, where it serves as an energy reserve. It is commercially available through Fresenius, it is neutral in charge, and does not undergo any non-renal elimination (4). However, inulin availability is limited and exogenous biomarker clearance study, either cold, or radiolabeled, have replaced inulin clearance studies. All of the newer methods have their own problems (3), but iohexol clearance is probably the best approach (5). Unfortunately, these methods are impractical for each clinic visit, and therefore endogenous markers are used to estimate GFR. For centuries, the only endogenous biomarker was serum creatinine (3). More recently, cystatin C has been established as an excellent alternative (6). The best estimation of GFR is achieved when serum creatinine and cystatin C are combined (3). For children, the new bedside Schwartz formula (developed in a USA cohort of children with CKD and subsequently referred to simply as the new or modified Schwartz formula) (7) is recommended; whereas in adults, the CKD Epidemiology Collaboration (CKD-EPI) formula based on cystatin C and serum creatinine (developed from large studies from different parts of the world and differing measured GFR methods) is endorsed through international guidelines (8). Unfortunately, while these approaches work well on a population level, there are clearly special populations in whom this approach does not work at all; for instance, serum creatinine is not measuring renal function in the newborn up to 72 hours after birth as it reflects maternal function (9). It is possible to define all the possible special populations and to choose appropriate approaches (10), however, in some populations such as oncology survivors, no eGFR approaches are reliable and direct measurement of GFR is indicated (11-13). Despite of the usefulness of eGFR estimation, all eGFR approaches have significant limitations and the search for more accurate tools continues. In that context, we are delighted to learn about a novel two-marker dextran-based injectate method that can be used to simultaneously measure plasma volume and GFR. It is a visible fluorescent injectate that can be used to measure GFR at the bedside. The paper was published as a rapid communication in Journal of the American Society of Nephrology (JASN) (14).
The study by Rizk et al.
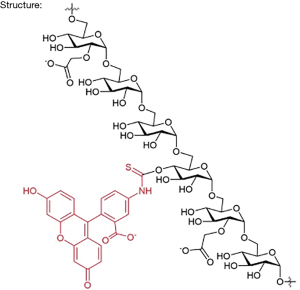
In the study by Rizk et al., the authors combine a large 150-kD rhodamine derivative and small 5-kD fluorescein carboxymethylated dextrans which shows a linear correlation with measured iohexol GFR in 32 adults (14). The authors call it visible fluorescent injectate (VFI). It is essentially a 2-photon in vivo fluorescence method (2 different molecular weight carboxymethyl dextran molecules (5 and 150 kD) with different fluorescent dye molecules attached) for optical measurement of GFR. The chemical structure will be similar to what is depicted in Figure 1.
Like Inulin, 5-kD fluorescein carboxymethylated dextrans are carbohydrate polymers that are commercially available. Through their National Institute of Health (NIH) well-funded research, the authors identified the best possible vehicle. The tolerability was tested in dogs first. There is not a lot of information available about this approach, but it is assumed that the small molecular weight compound is freely filtered in the glomerulus, similar to small molecular weight proteins and its volume of distribution is similar to the extracellular volume, and thus a GFR marker. The large compound will likely stay in the plasma compartment and reflect the circulating plasma volume. Like inulin, the dextrans are neutral in charge, however, the carboxyl groups will likely cause an overall negative charge of the molecule (15).
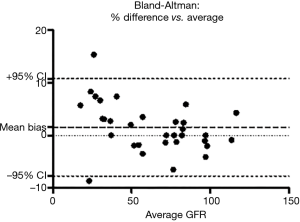
The compound has been found to be biocompatible and is currently explored as a starting material in several pharmaceutical and diagnostic applications (tdbcons.com/images/FITC-CM-dextran.pdf). The toxicity of the fluorescein carboxymethylated dextrans is also anticipated to be low. In the small study of Rizk et al., VFI was well tolerated. The authors found a strong correlation between the iohexol clearance and the VFI clearance (R2=0.9961). The mGFRs of the 32 patients were equally distributed across all stages of GFR. However, using gold standard Bland & Altman analysis, there was a mean bias of +4.733% with a 95% limit of agreement from −7.8% to +10.8%, which would be acceptable, but also a tendency to overestimate at lower GFR (Figure 2). We generated the Bland & Altman plot from the data that were available in Rizk’s manuscript.
Nonetheless, the results are indeed intriguing and may greatly enhance the utility of measuring rather than estimating GFR. The authors also showed an excellent reproducibility or within-subject variability of <5%. Moving from a two-point measurement to a three-point measurement and using non-linear two-compartmental models such as WinNonLin or NonMem may further improve the accuracy and reproducibility.
Future directions
So, are we going back to measured GFR as the older physicians used to employ in the 70s and 80s? The tolerability and safety as well as the accuracy need to be tested prospectively in large clinical trials. There are allergic reactions to inulin reported (16), and it is possible that the same may apply for VFI. The bias towards overestimation in the lower GFR range may be due to a lack of equilibration from the intravascular space to the extracellular volume, the likely volume of distribution. Reliable single shot exogenous GFR measurements require to wait at least 90–120 minutes to start sampling to allow for the necessary equilibration (3). The whole approach to have a FAST14 GFR measurement may not be feasible in the nephrotic state, advanced CKD with volume overload, or other conditions where the equilibration takes much longer. A Bayesian approach for the measurement using a lot more variables may be more feasible to obtain the FAST GFR measurement. The impact of the negative charge of the compounds requires further evaluation. Lots of work remains to be done, but a reintroduction of measured GFR at the bedside seems possible.
Acknowledgements
We congratulate Rizk and co-workers on this excellent research.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Filler G, Browne R, Seikaly MG. Glomerular filtration rate as a putative 'surrogate end-point' for renal transplant clinical trials in children. Pediatr transplant 2003;7:18-24. [Crossref] [PubMed]
- Soveri I, Berg UB, Bjork J, et al. Measuring GFR: a systematic review. Am J Kidney Dis 2014;64:411-24. [Crossref] [PubMed]
- Filler G, Yasin A, Medeiros M. Methods of assessing renal function. Pediatr Nephrol 2014;29:183-92. [Crossref] [PubMed]
- Toto RD. Conventional measurement of renal function utilizing serum creatinine, creatinine clearance, inulin and para-aminohippuric acid clearance. Curr Opin Nephrol Hypertens 1995;4:505-9; discussion 3-4. [Crossref] [PubMed]
- Schwartz GJ, Furth S, Cole SR, et al. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int 2006;69:2070-7. [Crossref] [PubMed]
- Filler G, Bokenkamp A, Hofmann W, et al. Cystatin C as a marker of GFR--history, indications, and future research. Clin biochem 2005;38:1-8. [Crossref] [PubMed]
- Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 2012;82:445-53. [Crossref] [PubMed]
- Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20-9. [Crossref] [PubMed]
- Filler G, Guerrero-Kanan R, Alvarez-Elias AC. Assessment of glomerular filtration rate in the neonate: is creatinine the best tool? Curr Opin Pediatr 2016;28:173-9. [Crossref] [PubMed]
- Filler G, Lee M. Educational review: measurement of GFR in special populations. Pediatr Nephrol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Bernhardt MB, Moffett BS, Johnson M, et al. Agreement among measurements and estimations of glomerular filtration in children with cancer. Pediatr Blood Cancer 2015;62:80-4. [Crossref] [PubMed]
- Laskin BL, Nehus E, Goebel J, et al. Cystatin C-estimated glomerular filtration rate in pediatric autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012;18:1745-52. [Crossref] [PubMed]
- Oc MA, Demir H, Cekmen MB, et al. Correlation of Cystatin-C and radionuclidic measurement method of glomerular filtration rate in patients with lung cancer receiving cisplatin treatment. Ren Fail 2014;36:1043-50. [Crossref] [PubMed]
- Rizk DV, Meier D, Sandoval RM, et al. A Novel Method for Rapid Bedside Measurement of GFR. J Am Soc Nephrol 2018;29:1609-13. [Crossref] [PubMed]
- Martin C, Dolmazon E, Moylan K, et al. A charge neutral, size tuneable polymersome capable of high biological encapsulation efficiency and cell permeation. Int J Pharm 2015;481:1-8. [Crossref] [PubMed]
- Gaspari F, Perico N, Remuzzi G. Measurement of glomerular filtration rate. Kidney Int Suppl 1997;63:S151-4. [PubMed]