Tumor stress-induced phosphoprotein 1 as a prognostic biomarker for breast cancer
Introduction
Breast cancer is one of the common cancer in Chinese females; cases in China are about 12.2% of all newly diagnosed breast carcinoma and about 9.6% of all deaths from breast cancer worldwide (1). Breast cancer is a heterogeneous tumor, of which the most frequent histological subtype is invasive ductal carcinoma, which accounts for 70% to 80% of all cases (2). Breast cancer may be further divided into four subtypes, as follows: the luminal A, luminal B, human epidermal growth factor receptor 2 (HER-2) over-expression and basal-like subtypes (3). Tumor metastasis occurs in more than 25% of breast cancer patients. Clinicopathologic characteristics such as tumor size, lymph node status, invasion of vessels, and hormone receptor status play important roles in the prediction of metastasis risk (4). New prognostic biomarkers for metastasis risk or overall survival (OS) (for example, protein, microRNA, lncRNA and epigenetic modifications) have been continually investigated (5). Classical clinical prognostic biomarkers such as estrogen receptor (ER), progesterone receptor (PR) and HER-2 have played a role in the identification of which patients are likely to benefit from endocrine therapy or targeted therapy (6,7). However, due to tumor heterogeneity, the current biomarkers that predict prognosis have some limitations, and thus, the field needs new biomarkers as prognostic indicators to effectively distinguish less aggressive cancers from aggressive cancers.
Stress-induced phosphoprotein 1 (STIP1, STI1, gene ID 10963), also known as heat shock protein 70/90 organizing protein, is a 62.6-kDa protein that comprise three tetratricopeptide repeat motifs and two nuclear localization signals (8). STIP1 pays important role in transcription, protein folding and translocation, cell division, signal transduction, and viral replication (9,10). Recent studies have suggested an important relationship between STIP1 and cancer. For example, STIP1 is over-expressed in a number of tumors, such as papillary thyroid carcinoma (PTC), pancreatic cancer, ovarian cancer and cholangiocellular carcinoma (11-14). Recent studies show that high STIP1 expression correlates with the prognosis of metastatic ovarian cancer and with a poor prognosis in PTC, though the function of STIP1 is little known (11,15).
To date, the expression of STIP1 in breast cancer tissues and its relationship with clinical characteristics and survival have not been investigated in humans. In this study, we investigate the expression of STIP1 protein in breast cancer specimens and adjacent normal tissues by immunohistochemistry (IHC) and explore its prognostic significance.
Methods
Patients
We have collected clinical data from 588 breast cancer patients, which were shown in our previous publication (16). Our previous work suggested that obese patients with breast cancer have poorer prognosis than normal weight one, and that overweight patients with breast cancer have comparable prognosis with normal weight one (16). In order to eliminate the interference of obese on the aim of the study, we randomly selected 228 normal weight or overweight (BMI 18.5–28 kg/m2) cases in which the expression of STIP1 was detected in breast cancer tissues and adjacent normal tissues by IHC. The 5-year recurrence-free survival (RFS) and OS rates according to high or low expression of STIP1 were also evaluated. The detailed information is summarized as follows: clinical operative specimens were gained from Pathology Department of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology from January 2008 to December 2010. The local ethics committee authorized us to use specimens for research (IRB ID: TJ-C20151107). All patients filled in informed consent that their tissues would be used to do research. We obtained patients’ information through telephone and clinic medical records in November 2015. The recruited patients met the following criteria: (I) patients’ age was over 18 years; (II) patients suffered from breast-conserving surgery (BCS) or modified radical mastectomy (MRM), accomplished standard adjuvant chemotherapy or neoadjuvant chemotherapy and did not relapse or die before radiotherapy and (or) chemotherapy at our hospital; (III) patients with clinical stage of I to III; (IV) patients had done reexamination every 3 months in the first 2 years and every 6 months since the third years after chemotherapy; (V) patients suffered from annual whole body ultrasonic scan including breasts, axillary fossa, abdomen, pelvis, and cervix, annual mammography and annual chest X-ray scan (17).
Patient follow-up
The followed contents were as follows: whether death or recurrence, the part and time of recurrence, and the date and cause of death. Recurrent events referred to the locoregional relapse (including chest wall, the ipsilateral breast and axillary and supraclavicular nodes, the skin close to the lesion and the surgical scar, and the internal mammary gland) and/or distant metastasis, such as bones, liver, lung, brain, and the contralateral breast. The 5-year RFS rate and OS rate were evaluated in high expression STIP1 group and low expression STIP1 group. The time of RFS was defined as the interval between the date of first surgical treatment and the date of the earliest recurrence within 5 years. The time of OS was defined as the interval between the date of the first surgical treatment and the date of death within 5 years.
IHC
The IHC was used to detect the expression of STIP1 protein in clinical specimens through a polyclonal antibody against STIP1 protein (ABclonal, Boston State, USA). The protocol of IHC was according to a previous publication (18). The immunohistochemical score was assessed independently through a blinded fashion which was on the base of the staining intensity and the proportion of positive cells. The score was from 0 to 7. Patients with score of 0 to 4 were regarded as low expression of STIP1 protein while with score of 5 to 7 were regarded as high expression of STIP1 protein (11).
Statistical analysis
The statistic difference of categorical variables was evaluated by chi-square (χ2) test (the Fisher exact test was performed when χ2 test was inappropriate). The 5-year RFS and OS curves and statistic difference were evaluated through the Kaplan-Meier method and the log-rank test. The hazard ratios (HRs) of STIP1 and other clinical variables that may be associated with replace and death were calculated by univariate and multivariate analyses employing a Cox regression model. The statistical analyses and curves were generated by SPSS software 22.0 (SPSS, Chicago, IL, USA). A P value less than 0.05 (two-sided) was regarded as having statistic difference.
Results
Expression of STIP1 was higher in breast cancer tissues than in adjacent normal tissues
The rate of high expression of STIP1 was 55.3% (126/228) in breast cancer tissues and 14.9% (34/228) in adjacent normal tissues. This difference is statistically significant (χ2=81.495, P<0.001). Representative immunohistochemical images were presented in Figure 1.
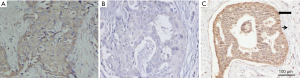
High expression of STIP1 was associated with tumor size, stage and HER-2 status
Significant differences were observed in tumor size, stage and HER-2 status, but no significant differences were observed in age, onset of menopause, histology, lymph node status, or ER and PR status between the high STIP1 expression group and the low STIP1 expression group (Table 1). The rate of high expression of STIP1 was 62.1% (72/116) in tumors larger than 2 cm and was 48.2% (54/112) in tumors smaller than 2 cm (χ2=4.424, P=0.035). The rate of high expression of STIP1 was 64.2% (52/81) in the stage III group and was 50.3% (74/147) in the stage I and II groups (χ2=4.056, P=0.044). The rate of high expression of STIP1 was 71.6% (48/67) in the HER-2 positive group and was 48.4% (78/161) in the HER-2 negative group (χ2=10.295, P=0.001).
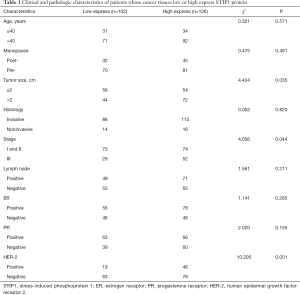
Full table
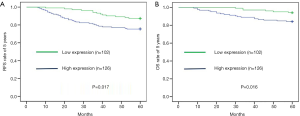
High expression of STIP1 in cancer tissues is associated with the poor prognosis of patients
Forty-four patients relapsed and 26 patients died for breast cancer within 5 years. The overall rate of relapse within 5 years was 19.3% while the overall rate of mortality within 5 years was 11.4%. There were 13 patients relapsed and 6 died in the low STIP1 expression group, while 31 patients relapsed and 20 died in the high STIP1 expression group. The 5-year RFS rate was 87.3% in the low STIP1 expression group and was 75.4% in the high STIP1 expression group; this difference was statistically significant (χ2=5.721, P=0.017) (Figure 2A). The 5-year OS rate was 94.1% in the low STIP1 expression group and was 84.1% in the high STIP1 expression group; this difference was statistically significant (χ2=5.814, P=0.016) (Figure 2B). The HRs of STIP1 and other clinical variables were shown in Table 2. There was a close correlation between high STIP1 expression and high rate of relapse for unadjusted HR was 2.161 (95% CI, 1.130–4.130). When adjusting other positive predictors, the adjusted HR of STIP1 was 1.983 (95% CI, 1.031–3.815). Besides, HER-2 status was a predictor of relapse as well. High STIP1 expression was also associated with high rate of death for unadjusted HR was 2.915 (95% CI, 1.170–7.259). When adjusting other positive predictors, the adjusted HR of STIP1 was 2.530 (95% CI, 1.003–6.379). Besides, HER-2 status was a predictor of death as well.
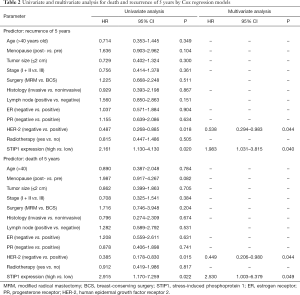
Full table
Discussion
Breast cancer is divided into several different subtypes, each of which has different molecular profiles, biological behaviors, and risk profiles (19). A markedly prognostic factor of breast cancer is the histological subtype; an example is invasive ductal carcinoma, which accounts for 70% to 80% of all cases (2). Unfortunately, the determination of the histological subtype is subjective for which was diagnosed by pathologists, and in particular, some cases do not conform precisely to a given subtype. One of the methods to recover the confusion is to class breast cancer according to molecular subtypes with different gene expression signatures which has been suggested to improve our understanding of the molecular basis of the histological subtypes (20). Effective prognostic and predictive biomarkers are important tools for individualized treatment, which distinguish patients with low-risk characteristics from those who are likely to experience unwanted side effects from over-treatment.
STIP1 was reported to be expressed in several types of tumors. It was reported that STIP1 expressed highly in ovarian cancer cells where it facilitates cell proliferation and migration (8). In our study, high expression of STIP1 was positively associated with tumor size and stage, which was similar to what was reported by Tsai et al. Similarly, one independent group used two-dimensional differential in-gel electrophoresis and by mass spectrometry-based label-free proteomics and reported that the expression of STIP1 was higher in cholangiocellular carcinoma than in normal hepatocytes and non-tumorous cholangiocytes (14). In our study, the expression of STIP1 was higher in breast cancer tissues than in adjacent normal tissues, which was in agreement with the results of Padden et al. Although the role of STIP1 in tumor pathogenesis has not been fully elucidated, the association between STIP1 and PTC has been reported (11). Yuan et al. reported that patients with higher STIP1 expression had a shorter OS time and suggested that STIP1 is an independent biomarker for the poor prognosis of patients with PTC. In our study, high STIP1 expression in breast cancer was associated with low 5-year RFS and OS rates, and STIP1 was an independent predictor of relapse and death. These results are consistent with those of Yuan et al.
The study suggested that STIP1 was a prognostic biomarker for breast cancer, so STIP1 may be a potential target for breast cancer treatment. Díaz-Chávez et al. have reported that MCF-7 breast cancer cells exposed to resveratrol (3’,4’,5-trans-trihydroxystilbilene) decreased the expression of STIP1 protein (21). Tsai et al. have reported that using STIP1-neutralizing antibody to eliminate STIP1 suppressed human ovarian cancer cells proliferation (8). STIP1 was also known as heat shock protein 70/90 organizing protein, Okada et al. have reported that DSCG (disodium cromoglycate) and amlexanox targeted Hsp90 to anti-allergic drugs (22). These studies suggested that resveratrol, STIP1-neutralizing antibody, DSCG and amlexanox may be potential drugs for breast cancers highly expressed STIP1.
The majority of breast cancers are ER-positive and respond to estrogens, and consequently, it is commonly treated with anti-hormonal therapy (3). In addition, the oncogene HER-2 has been identified as a predictor of patient prognosis (23). HER-2 positivity is strongly associated with breast cancer relapse and shorter OS (24). Hence, it is important to assess the HER-2 expression level in all breast cancer patients (25). We first showed the correlation between STIP1 expression and HER-2 expression in breast cancer. Based on our results, increased STIP1 expression levels are significantly associated with HER-2 positive expression, which indirectly indicates that high STIP1 expression is associated with a poor prognosis of breast cancer patients. Nevertheless, whether an interaction or regulation exists between HER-2 and STIP1 expression should be elucidated through further experiments.
There are two limitations in the study. Firstly, it was a retrospective study; secondly, the number of samples was relatively small. Thus, it needs a randomized study with enough samples to demonstrate that high expression of STIP1is associated with high rate of recurrence and death in breast cancer patients.
Conclusions
The results of our study have suggested that the histochemical scores of STIP1 may be serviceable to supplement the pathologist’s histopathological grade of breast cancers. In particular, the histochemical scores of STIP1 may be useful to predict the prognosis of breast cancer patients.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (No. 81372434) to H Xiong.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The local ethics committee authorized us to use specimens for research (IRB ID: TJ-C20151107) and written informed consent was obtained from all patients.
References
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Siziopikou KP. Ductal carcinoma in situ of the breast: current concepts and future directions. Arch Pathol Lab Med 2013;137:462-6. [Crossref] [PubMed]
- Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [Crossref] [PubMed]
- Ran S, Volk L, Hall K, et al. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology 2010;17:229-51. [Crossref] [PubMed]
- Adams BD, Wali VB, Cheng CJ, et al. miR-34a Silences c-SRC to Attenuate Tumor Growth in Triple-Negative Breast Cancer. Cancer Res 2016;76:927-39. [Crossref] [PubMed]
- Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer 2010;17:R245-62. [Crossref] [PubMed]
- Johnston SR. Clinical efforts to combine endocrine agents with targeted therapies against epidermal growth factor receptor/human epidermal growth factor receptor 2 and mammalian target of rapamycin in breast cancer. Clin Cancer Res 2006;12:1061s-68s. [Crossref] [PubMed]
- Tsai CL, Tsai CN, Lin CY, et al. Secreted stress-induced phosphoprotein 1 activates the ALK2-SMAD signaling pathways and promotes cell proliferation of ovarian cancer cells. Cell Rep 2012;2:283-93. [Crossref] [PubMed]
- Longshaw VM, Chapple JP, Balda MS, et al. Nuclear translocation of the Hsp70/Hsp90 organizing protein mSTI1 is regulated by cell cycle kinases. J Cell Sci 2004;117:701-10. [Crossref] [PubMed]
- Odunuga OO, Longshaw VM, Blatch GL. Hop: more than an Hsp70/Hsp90 adaptor protein. Bioessays 2004;26:1058-68. [Crossref] [PubMed]
- Yuan MH, Zhou RS, She B, et al. Expression and clinical significance of STIP1 in papillary thyroid carcinoma. Tumour Biol 2014;35:2391-5. [Crossref] [PubMed]
- Walsh N, O'Donovan N, Kennedy S, et al. Identification of pancreatic cancer invasion-related proteins by proteomic analysis. Proteome Sci 2009;7:3. [Crossref] [PubMed]
- Chao A, Lai CH, Tsai CL, et al. Tumor Stress-Induced Phosphoprotein1 (STIP1) as a Prognostic Biomarker in Ovarian Cancer. PLoS One 2013;8. [Crossref] [PubMed]
- Padden J, Megger DA, Bracht T, et al. Identification of Novel Biomarker Candidates for the Immunohistochemical Diagnosis of Cholangiocellular Carcinoma. Mol Cell Proteomics 2014;13:2661-72. [Crossref] [PubMed]
- Cho H, Kim S, Shin HY, et al. Expression of Stress-Induced Phosphoprotein1 (STIP1) is Associated with Tumor Progression and Poor Prognosis in Epithelial Ovarian Cancer. Genes Chromosomes Cancer 2014;53:277-88. [Crossref] [PubMed]
- Wu R, Liu T, Yang P, et al. Association of 15-hydroxyprostaglandin dehydrogenate and poor prognosis of obese breast cancer patients. Oncotarget 2017;8:22842-53. [PubMed]
- Li S, Yu KD, Fan L, et al. Predicting breast cancer recurrence following breast-conserving therapy: a single-institution analysis consisting of 764 Chinese breast cancer cases. Ann Surg Oncol 2011;18:2492-9. [Crossref] [PubMed]
- Celis JE, Gromov P, Cabezón T, et al. 15-prostaglandin dehydrogenase expression alone or in combination with ACSM1 defines a subgroup of the apocrine molecular subtype of breast carcinoma. Mol Cell Proteomics 2008;7:1795-809. [Crossref] [PubMed]
- Rivenbark AG, O'Connor SM, Coleman WB. Molecular and cellular heterogeneity in breast cancer: challenges for personalized medicine. Am J Pathol 2013;183:1113-24. [Crossref] [PubMed]
- Ma XJ, Salunga R, Tuggle JT, et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci U S A 2003;100:5974-9. [Crossref] [PubMed]
- Díaz-Chávez J, Fonseca-Sánchez MA, Arechaga-Ocampo E, et al. Proteomic profiling reveals that resveratrol inhibits HSP27 expression and sensitizes breast cancer cells to doxorubicin therapy. PLoS One 2013;8. [Crossref] [PubMed]
- Okada M, Itoh H, Hatakeyama T, et al. Hsp90 is a direct target of the anti-allergic drugs disodium cromoglycate and amlexanox. Biochem J 2003;374:433-41. [Crossref] [PubMed]
- Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther 2011;11:263-75. [Crossref] [PubMed]
- Pegram MD, Konecny G, Slamon DJ. The molecular and cellular biology of HER2/neu gene amplification/overexpression and the clinical development of herceptin (trastuzumab) therapy for breast cancer. Cancer Treat Res 2000;103:57-75. [Crossref] [PubMed]
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA):an open-label, randomised controlled trial. Lancet 2013;382:1021-8. [Crossref] [PubMed]


