Prognostic value of circumferential resection margin in T3N0M0 esophageal squamous cell carcinoma
Introduction
Surgery is still the cornerstone of the treatment of advanced esophageal cancer, and the most important principles of surgical resection of primary esophageal cancer is complete resection. Positive proximal and distal resection margins have repeatedly been reported to decrease the effect of operative resection (1,2). In contrast, the role of circumferential resection margin (CRM) in esophageal cancer is elusive for contrary results of previous studies on this topic (3).
The CRM represents the adventitial soft tissue margin closest to the deepest tumor penetration. Currently, there are two common criteria given the definition of CRM involvement. First, the College of American Pathologists (CAP) defines only a presence of tumor at the cut margin as CRM positive. Second, the Royal College of Pathologists (RCP) defines a positive CRM as a tumor at or within 1 mm of the circumferential cut margin (4).
One explanation for this discrepancy result may be the heterogeneity of the studies. The patients with mixed different T stage, various preoperative therapy (with or without neoadjuvant treatment), and inconsistent tumor node metastasis (TNM) classification version (5) contributed to the conflict results. In order to make further exploration of the exclusive role of CRM on ESCC patients, we only included the patients at pT3N0M0 stage and without receiving neoadjuvant radiochemotherapy in this study.
Methods
Patients
From June 2009 to July 2011, patients who underwent esophagectomy for esophageal squamous cell carcinoma (ESCC) at West China hospital of Sichuan University were retrospectively selected. In order to investigate the sole function of CRM, only pT3N0M0 patients were enrolled in this study. The additional inclusion criteria are complete resection (without microscopic tumor at the proximal and distal resection margin), and without surgical mortality (defined as death occurring within 30 days of operation). The Ethics Committees of West China Hospital of Sichuan University approved the study (No. 201649).
Operative procedure
Curative-intent resection were undergone in all patients. Approach of left thoracotomy (single incision) and Ivor Lewis were performed in patients with tumor in middle or lower thoracic esophagus and no evidence of lymph node involvement in the superior mediastinum or in the neck. McKeown approach was performed in patients with tumor in the middle or upper thoracic esophagus or with possible LN metastasis in the superior mediastinum or neck.
Pathologic examination
Multiple records of each patient were re-evaluated and modified by certified pathologists. When compared to the proximal and distal margins, the circumferential margin was defined as the lateral cut edge of the resected specimen, which is parallel to straight esophageal axis. The CRM status was judged by measuring the minimum distance from the tumor cells to the vertical margin. The CAP or RCP criteria was used to identify the CRM status including R0 and R1. The 8th edition of the American Joint Committee on Cancer TNM staging system was used to determine the pathologic stages of ESCC.
Statistical analysis
Chi-square or Fisher’s exact probability test was used to perform the comparisons of categorical data. Continuous variables were compared by two-tailed t test. Mann-Whitney U test was applied wherever required. Overall survival curve was computed by the Kaplan-Meier curve, and the log-rank test was used to compare the differences among survival curves. Univariate and multivariate analyses were performed by the Cox proportional hazards model. Variates with P values <0.20 in univariate analysis were entered into multivariate analysis. Optimal cutoff points for CRM were determined by minimum P value from log-rank χ2 statistics through the X-tile program (Version 3.1.2, Yale University). The prognostic performance of different classification of CRM was compared in terms of homogeneity, discriminatory ability, and monotonicity: (I) the likelihood ratio χ2 test related to the cox regression model was used to measure homogeneity; (II) the linear trend χ2 test was conducted to measure the monotonicity of gradients assessments; (III) Harrell’s c statistics was carried out to measure the discriminatory ability. In order to obtain the potential bias in comparing different classification, the Akaike information criterion (AIC) within the Cox proportional hazard model was used. The AIC was defined as: AIC = −2 log maximum likelihood ×2 (the number of parameters in the model). A smaller AIC value indicates a better model for predicting outcome. Harrell’s C index range from 0.5 (no discrimination for predicting OS) to 1.0 (perfect discrimination). All statistical analyses were carried out by SPSS, version 20.0 (SPSS Inc., Chicago, IL), and a P value of less than 0.05 was considered significant.
Result
A total of 112 patients were included in the study. The median follow-up time for all patients was 42.23 months (range, 2.6–87.7 months). The 1-, 3- and 5-year OS rates were 83.9%, 50.9%, and 44.9%, respectively. Patients were stratified into CRM positive and negative group based on CRP and RCP criteria. The characteristic and correlation of patients between CRM status and clinicopathological parameters were listed in Table 1.
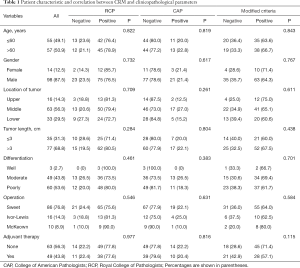
Full table
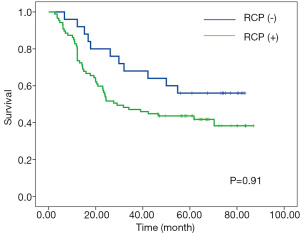
According to RCP, a positive CRM (CRM+) was identified in 87 patients (77.7%) with the median-survival time of 29.1-month; whereas CRM+ was achieved in 24 patients (21.4%) with the median-survival time of 24.3-month according to criteria of CAP. No significant associations between overall survival and CRM were observed according to neither CAP nor RCP criteria (Figures 1,2).
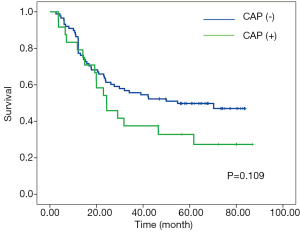
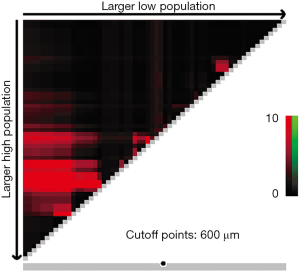
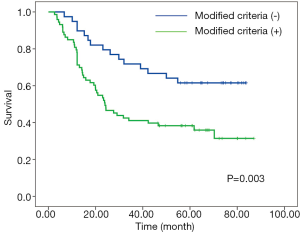
X-tile plots indicated that the optimal cutoff points for CRM were 600 µm from minimum P value of log-rank χ2 test (Figure 3). According to this modified CRM classification, CRM+ was identified in 39 patients (34.8%) with the median survival time of 23.9-month and was associated with a significant worse overall survival (P=0.003) when compared with CRM+ (Figure 4).
Apart from the three criteria, other clinicopathologic factors, such as age, gender, histologic grading, tumor location, pathological tumor length, adjuvant therapy were included in both univariate and multivariate analysis. The results showed that only the modified CRM criteria has significant effect on overall survival from multivariate analysis (Table 2).
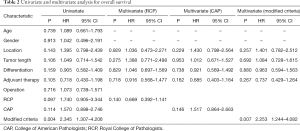
Full table
In addition, when compared with CAP and RCP, the modified CRM criteria (reclassified by 600 µm) showed improved homogeneity, monotonicity of gradients, and discriminatory ability (Table 3).

Full table
Discussion
This study revealed that a positive circumferential resection margin did not associate with a decreased overall survival according to neither the CAP nor RCP criteria. In order to emphasize the prognostic value of CRM, a modified stratification was proposed based on this cohort. A standard set of CRM definitions should be eligible to cover the following major aspects: (I) the difference in survival time is minimal among patients who are classified into the same group by that system (homogeneity); (II) as compared with this difference, patients classified into different groups have much greater survival time differences (discriminatory ability); (III) the mean survival time for a group classified as favorable by staging system is always longer than the survival times of groups classified as less than favorable (monotonicity) (6). Based on the above three evaluation criteria, we carried out multiple analyses which highlighted that the modified stratification had a better prognostic performance.
Some studies suggested that CRM involvement be a powerful prognostic indicator for ESCC, whereas others failed to find a significant influence of CRM involvement. (3,4,7-9) In fact, whether CRM of ESCC should be recognized as an independent prognostic factor for survival or not is a controversial issue in clinical research (10). As mentioned above, the heterogeneity on T stages could partly explain for the discrepancy result, since that much of the previous studies included patients from T0–T4 stage. On the one hand, the most pertinent population for comparison should be the patients at T3 stage, and CRM involvement is not significant in patients with early (T1 or T2) or advanced (T4) tumor (3). On the other hand, T1 or T2 tumor with CRM involvement is surgical failure and should not be mixed up with the T3 tumors (9). Another considerable heterogeneity should be the lymph node involvement, since lymph node status has been shown to be a strong independent prognostic factor in patients with esophageal cancer (11,12). Given that CRM+ was more frequently found in ESCC patients with lymph node metastasis (3,4,9), a mixed population with varied lymph node status would subsequently confound the role of CRM in ESCC. In order to eliminate such confounded factors, our study only included patients at pT3N0M0 stage. To our best knowledge, the present study is the first study to evaluate the individual prognostic value of CRM through merely selecting ESCC patients with negative lymph node.
When compared with the existed conflicting evidence about CRM involvement as a prognostic marker in ESCC patients, CRM status has been a well-established predictor of local recurrence and long-term survival in rectal cancer (13,14). The histo-anatomical heterogeneity may be responsible for the different oncological outcomes and impacts of CRM in either esophageal or rectum cancer. In detail, the rectum is surrounded by a physical barrier constructed by mesorectum, but the esophagus is surrounded without a real anatomic barrier. The absence of serous layer will facilitate the local invasion to the adjacent structures and tissue, which in return, revealing more positive CRM in esophageal cancer.
Several limitations to this study should be mentioned, such as its retrospective work with observational data. The CRM was not measured by a single specialist. An inter-observer bias, therefore, may exist. The Sweet procedure is the predominant type of operation. Since its limitation in the extent of lymphadenectomy as compared with Ivor-Lewis and McKeown, the false-negative lymph nodes maybe happen to some patients. Furthermore, there lacks validation within an external patients cohort in order to assess accuracy and calibration of this novel stratification. Future studies will be required to ascertain the prognostic significance of criteria based on the results of this study.
In summary, our study suggested that 600 µm should be considered as an optimal cut-off point of CRM for prognostic evaluation of patients with ESCC. Prospective studies may be warranted to further validate the prognostic performance of this modified criteria.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committees of West China Hospital of Sichuan University approved the study (No. 201649).
References
- Wang YC, Deng HY, Wang WP, et al. Positive esophageal proximal resection margin: an important prognostic factor for esophageal cancer that warrants adjuvant therapy. J Thorac Dis 2016;8:2512-8. [Crossref] [PubMed]
- Mariette C, Castel B, Balon JM, et al. Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur J Surg Oncol 2003;29:588-93. [Crossref] [PubMed]
- Ghadban T, Reeh M, Koenig AM, et al. Prognostic Significant or Not? The Positive Circumferential Resection Margin in Esophageal Cancer: Impact on Local Recurrence and Overall Survival in Patients Without Neoadjuvant Treatment. Ann Surg 2017;266:988-94. [Crossref] [PubMed]
- Lee GD, Lee SE, Kim KM, et al. New 3-Tiered Circumferential Resection Margin Criteria in Esophageal Squamous Cell Carcinoma. Ann Surg 2015;262:965-71. [Crossref] [PubMed]
- Karstens KF, Izbicki JR, Reeh M. Does the Margin Matter in Esophageal Cancer? Dig Surg 2018;35:196-203. [Crossref] [PubMed]
- Yoon HM, Ryu KW, Nam BH, et al. Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg 2012;214:88-96. [Crossref] [PubMed]
- Hulshoff JB, Faiz Z, Karrenbeld A, et al. Prognostic Value of the Circumferential Resection Margin in Esophageal Cancer Patients After Neoadjuvant Chemoradiotherapy. Ann Surg Oncol 2015;22 Suppl 3:S1301-9. [Crossref] [PubMed]
- Okada N, Fujii S, Fujita T, et al. The prognostic significance of the positive circumferential resection margin in pathologic T3 squamous cell carcinoma of the esophagus with or without neoadjuvant chemotherapy. Surgery 2016;159:441-50. [Crossref] [PubMed]
- Depypere L, Moons J, Lerut T, et al. Prognostic value of the circumferential resection margin and its definitions in esophageal cancer patients after neoadjuvant chemoradiotherapy. Dis Esophagus 2018.31. [PubMed]
- Wu J, Chen QX, Teng LS, et al. Prognostic significance of positive circumferential resection margin in esophageal cancer: a systematic review and meta-analysis. Ann Thorac Surg 2014;97:446-53. [Crossref] [PubMed]
- Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008;247:365-71. [Crossref] [PubMed]
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [Crossref] [PubMed]
- Galandiuk S. Standardization or Centralization: Can One Have One Without the Other? Circumferential Resection Margins and Rectal Cancer. Ann Surg 2015;262:899-900. [Crossref] [PubMed]
- Rickles AS, Dietz DW, Chang GJ, et al. High Rate of Positive Circumferential Resection Margins Following Rectal Cancer Surgery: A Call to Action. Ann Surg 2015;262:891-8. [Crossref] [PubMed]