Improved identification of secondary hypertension: use of a systematic protocol
Introduction
Secondary hypertension (SH) is best defined as a form of hypertension with an identifiable cause and is generally considered to affect approximately 5–10% of all hypertensive patients (1,2). Nevertheless, in a comprehensive review of published work, there were significant variations of the estimated prevalence of SH, ranging from 2% to 20% depending on the underlying etiology leading to high blood pressure (HBP) and the availability of adequate diagnostic laboratory and imaging resources (1-6). The recent 2017 American College of Cardiology/American Heart Association (ACC/AHA) High Blood Pressure Guideline recommends screening for a specific form of hypertension when clinical indications and physical examination findings are present, or in adults with resistant hypertension (7). While many diagnostic tools exist to assess SH, physicians and other healthcare providers who treat hypertension may find it difficult to detect due to the multiple etiologies and suboptimal recognition of the condition.
In 2002, the Center of Hypertension was created at the Institute of Cardiology (CHIC), at Austral University Hospital in Buenos Aires, Argentina. The Center’s activities are characterized by an intensive interplay between patient care, teaching and clinical-translational research. As a teaching and referral unit, the Center created a systematic and standardized approach to screen patients with hypertension in search for an identifiable cause for their HBP, based on contemporary guideline recommendations and local expertise (7-9). Utilizing the methods developed in the Center, this approach, known as the CHIC Protocol, could constitute a valuable tool for early recognition and accurate diagnosis of SH in clinical practice.
In 2017, we analyzed patient data from the preceding decade and screened for SH using this Protocol, identifying a higher level in the prevalence of SH among our patients, compared to previously published world-wide reports (1-9). Although secondary causes of hypertension are common in patients with resistant hypertension, a higher prevalence of SH persisted independent of the measured severity of BP values and SH was also identified in patients with moderate hypertension. Based on these results, we propose that our approach, originally developed for the unique diagnostic evaluation for physicians at the Institute of Cardiology, may represent a methodology that would facilitate the identification of SH in a variety of clinical sites.
Methods
We conducted a comprehensive search of medical records of 28,633 consecutive hypertensive patients evaluated at the Center of Hypertension, Institute of Cardiology at Austral University Hospital from January 1, 2007 to January 1, 2017. During that period, all attending physicians were instructed to classify diagnoses utilizing the International Code of Diseases (ICD9), assigning 401 to indicate essential (primary) hypertension and 405 for SH. Institutional medical records were digitized, provided by the PECTRA-BPM software and adapted for Electronic Health Record or Open EHR. For eligibility, patients were required to be 21 years old or greater at the time of the initial diagnosis, along with a complete and comprehensive medical record available for evaluation. Permission was obtained from Institutional Ethics and Review Board for the revision of clinical records, the development of our database and results analysis.
Participants were classified as having treatment resistant hypertension (TRH) or non-treatment resistant hypertension (NTRH), according to the contemporaneous definitions during the period analyzed. Accordingly, using contemporary diagnostic criteria during that period, TRH is defined as BP above goal in spite of the concurrent use of 3 antihypertensive agents of different classes prescribed at optimal dosages, with ideally a diuretic as one of the agents (10). Classifying the hypertension as resistant identifies patients who are at high risk of having SH due to persistently high BP levels and who may benefit from special diagnostic and therapeutic considerations. Moreover, patients whose BP is controlled, but requires 4 or more drugs to do so are also considered as having TRH (10). Additionally, the NTRH cohort included patients with controlled BP with up to 3 drugs (7-9).
Data analysis was made through univariate analysis and proportional rates.
The CHIC protocol: a systematic questionnaire
The CHIC Protocol consists of related blocks of questions evaluating presenting symptoms and patient history to properly identify SH (Table 1). These blocks of questions are related to a specific etiologies of SH, and when answered affirmatively are suggestive for specific causations of the conditions. Aptly, physicians were instructed to apply the CHIC Protocol during the consultation and complete the questionnaire based upon these observations, interviews, and physical examinations.

Full table
The administration of the Protocol begins prior to the initiation of formal consultation, which is crucial in searching for signs of SH through the observation of patient facies and body habitus. Although over one hundred questions are included in CHIC Protocol, a physician need only utilize approximately ten minutes to complete a full evaluation. If any question is responded to affirmatively, it is formerly re-evaluated to analyze for the risk of SH and to determine, following medical evaluation, which specific laboratory analyses and/or imaging tests are needed.
Results
A final population of 12,284 patients with hypertension was included in this study (mean age 59.9; SD, 19 years, 41% male, 59% female). We classified participants into two groups, based upon their anti-hypertensive treatment response: a cohort diagnosed with NTRH (7,617 patients, 62%) and the other with TRH (4,667 patients, 38%) (Figure 1). SH was documented in 41.8% (5,134 patients) of the total population.

Of the patients identified as having SH, 53.6% [2,759]patients belonged to the NTRH group and 46.3% [2,380] were TRH patients (Figure 1). Specifically, when determining the prevalence of SH based on the category of NTRH and TRH groups, SH was identified in the NTRH and TRH groups at 36% and 50.9%, respectively. The causes of SH and their distribution among participants are shown in Table 2. Sex, age and body mass index (BMI) were not significantly different between NTRH and TRH groups.
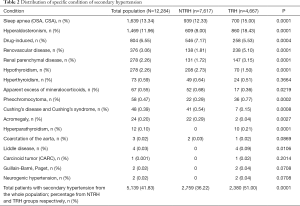
Full table
While the CHIC Protocol reflects a methodology to identify the risk of SH, physicians were encouraged to use confirmatory laboratory and imaging techniques for the diagnosis of the causes of SH. In consideration of the need for confirmatory diagnoses, we employed widely known procedures to detect in patients each etiology of SH, including, but not limited to, plasma renin activity, aldosterone levels, urinary metanephrine-normetanephrine concentrations, urinary vanillylmandelic acid concentrations, somatotropin levels, thyroid hormone levels and function testing, cortisol testing in saliva, blood and urine, adrenocorticotropic hormone test, Doppler ultrasound imaging, magnetic resonance imaging, computed tomography scanning, respiratory polysomnography and genetic testing for Liddle disease.
Discussion
Our results illustrate the prevalence and distribution of the causes of SH using the CHIC Protocol, revealing a higher percentage of SH than previously reported (1-6). The original aim during the development of this questionnaire was to generate a uniform protocol for the diagnosis of SH within an expert center. However, we consider that the unexpected higher prevalence of SH we revealed indicates the potential benefit for wider dissemination of our protocol in other facilities for the diagnosis of SH.
A 1976 review of historical and contemporary literature by Berglund et al. focused mainly on higher values of HBP and severe hypertension as a means to recognize the presence of SH. They described the prevalence of primary and SH in a population sample of 7,455 Swedish men aged 47 to 54 (10). The authors identified 798 men with BP above 175/115 mmHg during preliminary screening and recalled them for further evaluation. Among these patients with unusually severe hypertension, 3–6% had renal parenchymal disease, 6% had renovascular disease and 1–6% of patients were described as having other forms of SH. This investigation led to surgical treatment in only two cases. The authors concluded that for low prevalence of SH, especially for those that are curable with surgery, does not support the need for routine screening (10). Nevertheless, as opposed to these data and subsequent recommendations, we described a significantly higher prevalence among patients with a moderate level of hypertension. If the generalized concept that associates SH mainly with severe, resistant hypertension remains unchanged, many patients as described in our population would never be diagnosed.
Rimoldi et al. published a detailed review revising the true prevalence of SH by classifying hypertensives as NTRH and TRH, using techniques similar to the methodology in this study (1). Compared to the prevalence of SH in TRH patients reported by Rimoldi, we detected a similar prevalence of hyperaldosteronism (17% vs. 6–23%), pheochromocytoma (0.77% vs. <1%), but a lower prevalence of patients with obstructive sleep apnea (15% vs. >30%) (1).
By analyzing the causes and distribution of SH, we found that hyperaldosteronism is the most frequent endocrine etiology. However, our reported prevalence, while similar to that noted by others for TRH patients, revealed a higher prevalence of hyperaldosteronism in the NTRH cohort. This suggests that hyperaldosteronism may be hidden in controlled patients (11-13). The diagnosis of hyperaldosteronism in our population was based upon increased aldosterone-to-renin ratio, in efforts to avoid over-diagnosis of incidentaloma, according to guideline recommendations (1).
Although sodium intake was not evaluated in all patients in this study, we have previously shown that there was elevated dietary sodium in a sample from the same cohort (14). There was also an increase in the prevalence of patients with drug-induced hypertension, commonly due to the use of prescription and over-the-counter medications, including non-steroidal anti-inflammatory drugs, antacids and psychiatric drugs. In addition, associated BP elevation was seen with non-pharmacological agents such as natural laxatives, vitamins, and large doses of caffeine in energy drinks or “fat-burning” supplements.
In 2010, a review by Viera et al. assigned classic clues suspicious of SH to the best-achieved evidence available, including consensus, disease-oriented evidence, usual practice, expert opinion, or case series (15). The authors also classified the risk and causes of SH by age groups. From birth to 18 years old, it was suggested to investigate for renal parenchymal disease and coarctation of the aorta. In patients aged between 19 and 39, renovascular disease caused by fibrodysplasia and thyroid dysfunction was considered. For participants aged 40 to 64, aldosteronism, obstructive sleep apnea, pheochromocytoma, Cushing’s disease and Cushing’s syndrome was examined. Lastly, renal and renovascular disease and renal failure was analyzed in patients over 65 years of age (15). We did not identify the same age-related distribution of SH for patients older than 65 years old, but aldosteronism, obstructive sleep apnea, renovascular disease and thyroid dysfunction were frequently present in patients between aged between 40 and 70 years old.
Although the reported prevalence in published literature of secondary causes of hypertension has been identified to be 5% to 10% of all hypertensive patients, these rates of SH are significantly higher than the percentages identified in usual clinical practice. Under-recognition of SH in usual patient care could be due to obstacles regarding the awareness of SH and complementary methods for its diagnosis. Furthermore, factors that reduce the diagnosis of SH include the associated costs, under-recognition of clinically suggestive symptoms and the absence of systematic protocols. In addition, guidelines of scientific societies usually do not recommend its investigation in all hypertensive patients.
While it is reasonable that conventional practice and current guidelines attempt to avoid unnecessary costs in the pursuit of the diagnosis of SH, including testing for neurohormonal studies, suppression-stimulation tests or imaging, we suggest that using a systematic, standardized protocol as described herein, justifies an approach that does not abandon the search for SH in all patients. On the contrary, the CHIC Protocol may represent an opportunity to increase recognition of SH through anamnesis (or a detailed account of a patient’s medical history) and physical examination in all hypertensive patients. We consider our approach an inexpensive tool that primary care physicians and other healthcare providers can use prior to the initiation of formal consultation for an accurate reflection of the patient’s risk for SH. It is important to note that steps for the early recognition of SH using the CHIC Protocol require a straightforward evaluation prior to assessment by a hypertension specialist. Acquiring information using the CHIC Protocol represents a systematic approach to diagnose SH, facilitated by the application of this structured anamnesis, even if done by a non-specialist.
Overall, we have not revealed any novel diagnostic laboratory tests for the detection of SH, but have described the usefulness of applying what has been already shown to be beneficial: a systematic protocol that can be utilized in a broad range of hypertensive patients. Therefore, this approach can be a significant change of our diagnosis paradigm and could contribute to the appropriate recognition and diagnosis of SH in a wider range of patients with moderate and severe hypertension.
The main limitations of our study include its retrospective character and the associative relationship of certain etiologies for SH that have not been shown to have a causative role in all cases. Therefore, to positively assume that a condition is the direct cause of the development of hypertension, its treatment must be accompanied with a cure for the diagnosed SH. Further studies, including follow up evaluations are imperative to elucidate this dilemma.
Provider approaches for the application of the questionnaire
Application of the CHIC Protocol can be completed through physical observation of the patient during the consultation, as well as systematic questioning that can uncover critical components of their medical history. During the initial greeting you can perceive the patient’s hand temperature, and if it is sweaty, fragile, or has tremors, consider possible disorders such as Cushing’s syndrome, pheochromocytoma, thyroid dysfunction, scleroderma, or cerebrovascular disorders. While the consultation begins, dedicate a few seconds to carefully look at the facies for relevant data, such as the presence of prominent ciliary and jaw arches, with flattened nose and separated teeth in a large patient of either sex, in whom an acromegaly could be suspected. These suspicions may prompt for the investigation of other signs of acromegaly later in the consultation, such as galactorrhea, early visual disturbances, and polyuria. Suspect Cushing’s disease or Cushing’s syndrome if the patient has a rounded face, with ruddy cheeks and prominent abdomen, but with very thin and fragile legs. Exophthalmia may be present in patients with thyroid disorders or hypercortisolism. Pallor in a thin person, with a history of panic attacks may indicate pheochromocytoma. Hirsutism in women can identify patients with hyperandrogenism or mineralocorticoid receptor stimulation.
Revealing the family history to identify hereditary conditions that could lead to SH, such as Liddle’s disease, polycystic kidney disease or pheochromocytoma is critical. Liddle's disease, as well as pheochromocytoma, although rare in clinical practice, should always be considered in the anamnesis due to the high mortality associated with both conditions. These conditions often lead to events such as fatal myocardial infarction and stroke, where etiological diagnosis is made after autopsy. Liddle's disease usually manifests in childhood or adolescence, however, if BP is not measured, uncontrolled hypertension can go unnoticed and the patient may only note occasional myalgia or asthenia that could be underestimated during the first years of treatment.
If the patient is first diagnosed with hypertension in adulthood with hypokalemia, consider hyperaldosteronism. Similarly, if hypokalemia is present in a patient on diuretic therapy, clinicians should not be confused with diuretic effects that may mask or mitigate the presence of this condition. If hyperaldosteronism is suspected, the laboratory data may be misinterpreted in view of reduced renin and aldosterone associated with metabolic alkalosis.
Personal history and the search for pheochromocytoma
In hypertensive women who have had deliveries, it is important to obtain a history of hyperglycemia or gestational diabetes, which may be a manifestation of pheochromocytoma. Other antecedents of suspicion are syncope, hypertensive crisis during defecation or urination, or the presence of nocturnal headaches that awaken the patient and subside after urination. Headaches of the hypercatecholamine state are usually bilateral and pulsatile and hypercatecholamine crises of pheochromocytoma are sufficiently characteristic to not be confused with panic attacks. In suspected cases, evaluating a urine sample during a panic attack for urinary catecholamines is helpful, and it is of value to investigate the presence of triggers such as alcohol intake, menstrual periods, or the use of anesthetics.
In addition, arrhythmias can be key, although it is infrequently associated with SH with electrolyte disorders such as hypokalemia (hyperaldosteronism or hyperproduction of ACTH, Liddle’s syndrome or pheochromocytoma).
Systematized questioning
Asking targeted question is a great strategy when assessing SH using the CHIC Protocol, as it may identify risk factors that will not be elucidated through patient observation alone. For example, birth weight has been associated with an increased risk of nephropathy and premature aging. Additionally, the patient's weight history may lead to a pheochromocytoma if associated with progressive weight loss. The search for obstructive sleep apnea is important in obese subjects through questioning about their patterns of rest, snoring and morning sleepiness. However, its diagnosis usually goes unnoticed in non-obese hypertensive patients who have had otorhinolaryngological disorders associated with predisposing anatomical conditions, trauma in athletes, rhinoplasty, and some alterations in central regulation linked to cerebrovascular diseases. Not only is the patient’s nocturnal rest pattern important to determine in obese or thin patients alike, but it is also useful to interrogate about the characteristics of diuresis, such as polyuria, foam in the urine, pollakiuria, dysuria or recurrent infections.
The diagnosis of neurogenic hypertension is rarely suspected, but it may explain hypertension associated with stress, especially accompanied by hyperventilation. A simple maneuver is to measure the respiratory rate in acute or chronic stressful conditions. The history of syncope in a hypertensive patient also increases the suspicion of autonomous imbalances that must be accompanied with the evaluation of changes in heart rate, with standing to differentiate from orthostatic hypotension, dysautonomia and pheochromocytomas. Lastly, situations where there is an increase in intracranial pressure can also cause hypertension such as with cerebral infarction, brain tumors, diabetes insipidus, diabetes mellitus, optic atrophy, and deafness (DIDMOAD syndrome).
The initial questioning is also a reliable source of the data necessary to establish the diagnosis of hypertension secondary to pressor effects of drugs or interventions. For instance, a patient with COPD or immunological diseases may receive corticosteroids chronically. Nevertheless, it is unlikely for low-dose corticosteroids to cause HBP and although chronic use could be linked to some degree of a pressor, it may not be necessary to suspend these drugs for the control of BP when they are clearly indicated and necessary. The list of prescribed or over-the-counter drugs associated with hypertension are too numerous to list and beyond the scope of this comment, but it is recommended to remain alert for the consumption of any substance that may be promoting the increase in BP. The sodium content of food is essential to determine risk for HBP and adherence to sodium restriction, with education regarding the hidden sodium in foods is essential.
Some relevant signs in the physical examination
Lastly, there are important points to note regarding relevant signs during the physical examination, which may suggest SH. It is important to advance the examination from the feet to the head, since palpation of the pulses and detection of asymmetry may lead you to suspect the presence of aortic coarctation. The temperature and humidity of the extremities during the handshake at the beginning of the consultation, as well as any observed edema may be associated with disorders in the regulation of sodium in nephrogenic causes and hyperaldosteronisms. At this point, the patient may refer to additional history about joint problems that will add to the suspicion of acromegaly and Cushing’s. If stretch marks are observed on the abdomen, careful auscultation is important for renal murmurs that may be attributed to fibrodysplasia in young women or to atherosclerotic renovascular disease in older persons with proatherogenic factors. Finally, café-au-lait spots may trigger the suspicion of multiple endocrine tumors, as well as hirsutism and an increase of hair on arms or abdomen may signal states of hyperandrogenism.
In the general assessment, other secondary causes should be considered, including conditions associated with endocrine disorders such as vitamin D deficiency, obesity, male hypogonadism, and hyperparathyroidism.
Acknowledgements
We thank Dr. Alejandro Hita, and all the members of the Institute of Cardiology from the Austral University Hospital for their team working by allowing us to evaluate their patients; we would also like to thank Dr. Ezequiel Huguet, Patricia Carrizo, Patricia Lima, Mariana Haehnel, Laura Brandani, for their assistance in the evaluation of this large cohort of patients in Santa María de la Salud, and we thank all the staff that have collaborated by evaluating their patients into the Hypertension Center of the Austral University Hospital.
Footnote
Conflict of Interest: Dr. Ferdinand is a consultant for Amgen, Sanofi, Quantum Genomics, Novartis and Boehringer Ingelheim. Dr. Carol Kotliar often gives lectures for Novartis, Boehringer Ingelheim, Baliarda, Elea, Abbott, Gador and Menarini. The other authors have no conflicts of interest to declare.
Ethical Statement: Ethical approval to the use of an authorized data base and the protocol of the study was obtained from the Institutional Review Board at the Austral University and the Department of Academic Development from the Austral University Hospital. CIE (Committee of Institutional Evaluation) number 14-055.
References
- Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J 2014;35:1245-54. [Crossref] [PubMed]
- Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens 1994;12:609-15. [Crossref] [PubMed]
- Börgel J, Springer S, Ghafoor J, et al. Unrecognized secondary causes of hypertension in patients with hypertensive urgency/emergency: prevalence and co-prevalence. Clin Res Cardiol 2010;99:499-506. [Crossref] [PubMed]
- Omura M, Saito J, Yamaguchi K, et al. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res 2004;27:193-202. [Crossref] [PubMed]
- Sinclair AM, Isles CG, Brown I, et al. Secondary hypertension in a blood pressure clinic. Arch Intern Med 1987;147:1289-93. [Crossref] [PubMed]
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008;51:1403-19. [Crossref] [PubMed]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127-248. [Crossref] [PubMed]
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159-219. [Crossref] [PubMed]
- Consejo Argentino de Hipertensión Arterial. “Dr. Eduardo Braun Menéndez”. Sociedad Argentina de Cardiología. Consenso de Hipertensión Arterial. Rev Argent Cardiol 2013;81:1-72.
- Berglund G, Andersson O, Wilhelmsen L. Prevalence of primary and secondary hypertension: studies in a random population sample. Br Med J 1976;2:554-6. [Crossref] [PubMed]
- Calhoun DA. Is There an Unrecognized Epidemic of Primary Aldosteronism? Pro. Hypertension 2007;50:447-53. [Crossref] [PubMed]
- Tiu SC, Choi CH, Shek CC, et al. The use of aldosterone-renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different conditions of blood sampling. J Clin Endocrinol Metab 2005;90:72-8. [Crossref] [PubMed]
- Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 2004;89:1045-50. [Crossref] [PubMed]
- Kotliar C, Kempny P, Gonzalez S, et al. Lack of RAAS inhibition by high-salt intake is associated with arterial stiffness in hypertensive patients. J Renin Angiotensin Aldosterone Syst 2014;15:498-504. [Crossref] [PubMed]
- Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician 2010;82:1471-8. [PubMed]


