Stereotactic body radiation therapy for lung, spine and oligometastatic disease: current evidence and future directions
Management of early-stage non-small cell lung cancer (ES-NSCLC)
Lobectomy with mediastinal lymph node sampling remains the gold standard treatment of ES-NSCLC (AJCC stage I, TNM stage T1–2N0M0) with a 5-year overall survival (OS) of 35–97% and locoregional recurrence rates of less than 10% (1-3). However, many patients with ES-NSCLC are high-risk surgical candidates due to respiratory and cardiovascular comorbidities. Patients unable to undergo safe resection or who refused surgery were historically offered conventional external beam radiotherapy (cEBRT) [45–70 Gray (Gy) in 1.5–2.75 Gy fractions over up to seven weeks] or were observed without active cancer treatment. Over half of patients receiving observation alone ultimately die of cancer (4) with a median OS of 9 months (5), highlighting the need for active treatment in this population. However, outcomes with radiotherapy in this group are disappointing with a 3-year OS of 17–55% and local failure rates of 6–70% (6).
Stereotactic body radiation therapy (SBRT) for medically inoperable peripheral ES-NSCLC
SBRT is now established as a safe and effective option for treating medically inoperable peripheral ES-NSCLC (≤5 cm) endorsed by ESMO and NCCN as the treatment of choice in this setting (7,8). Peripheral tumors have been defined as being greater than 2 cm in all directions from the proximal bronchial tree (9,10) or any mediastinal critical structure (11).
Phase II studies have consistently produced local control (LC) rates in this patient group of approximately 90% at 3–5 years (12-14) with OS of 55–60% at 3 years (12,13) reflecting the medical comorbidities of the inoperable patient population and the development of subsequent metastatic disease. In a large single-center retrospective analysis of 676 patients receiving SBRT for ES-NSCLC with a median follow up of 33 months (14) two distinct patterns of recurrence emerged. The most common was isolated distant recurrence, occurring in 8% of patients at a median of 8.3 months after SBRT, suggesting the presence of subclinical disease at the time of treatment undetected by staging investigations. Isolated loco-regional recurrence (LRR) without distant metastases (a potentially salvageable situation) occurred in 6% at a median of 13.1 months from SBRT. In addition, 6% of patients developed a second lung primary cancer. The risk of recurrence or second lung primary was highest in the first 3 years after treatment, with many patients offered further curative treatment. To identify relapses or new primary cancers at an early-stage, consensus guidelines recommend computed tomography (CT) imaging after SBRT at 3, 6, 12, 18, 24, 36, 48 and 60 months (15).
Multiple dose fractionation regimes have been employed by centres developing SBRT, ranging from 1 to 10 fractions, usually delivered daily or on alternate days. There is no international agreement on the optimal SBRT regime for peripheral ES-NSCLC, but there does appear to be a dose range which produces adequate LC with acceptable toxicity. Retrospective analysis suggests both LC and OS are improved when the biological effective dose (BED) by the linear quadratic (LQ) model is >100 Gy delivered to the periphery of the tumor (assuming α/β of 10 Gy for tumor) (16). A meta-analysis of 34 observational studies identified inferior OS in regimes with a BED10 of >146 Gy (17); suggesting further dose escalation may not be beneficial. Caution should be exercised when comparing SBRT regimes using BED models due to the limitations of the LQ model at high doses per fraction, but this remains the most frequently used approach in clinical practice. Commonly employed regimes with a BED10 ≥100 Gy and the equivalent dose in 2 Gy fractions (EQD2) are shown in Table 1.
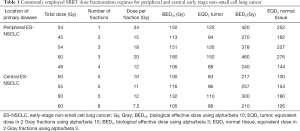
Full table
Toxicity
The high BED delivered with SBRT raises the concern of treatment-related toxicity, so tight margins, image guidance and a steep dose gradient are employed to minimise dose to normal structures. Using this approach acute toxicity is low and deterioration in health-related quality of life (HRQOL) is uncommon (18). The most commonly reported toxicities are pneumonitis and chest wall toxicity (10).
Pneumonitis
In a large pooled analysis of 505 patients receiving SBRT for ES-NSCLC with a variety of dose fractionation regimes (20–64 Gy/1–15 fractions), pneumonitis grade ≥2 and grade ≥3 developed in 7% and 2% of patients respectively (19). One case (0.2%) of fatal pneumonitis was reported. Median time to pneumonitis was 5 months. Symptomatic pneumonitis is usually self-limiting or resolves with corticosteroid treatment. Lagerwaard et al. (20) prospectively collected patient-reported HRQOL data for 382 consecutive patients treated with SBRT for ES-NSCLC. There was no significant change in dyspnea over 2 years, despite high pre-treatment symptom burden scores and 84% of patients having a diagnosis of chronic obstructive pulmonary disease (COPD). On retrospective analysis symptomatic pneumonitis/fibrosis is associated with older age, larger tumor size, higher mean lung dose and higher lung V20 (percentage of lung volume receiving over 20 Gy) (21). However, SBRT remains safe and well tolerated in the elderly (22-25) and those with COPD (26) and neither abnormal baseline pulmonary function tests (PFTs) nor age should preclude treatment (10). RTOG 0236 (54 Gy/3 fractions), reported a 6% reduction in forced expiratory volume in 1 second (FEV1) and diffusion capacity for carbon monoxide (DLCO), with minimal change in arterial blood gases and no significant decline in oxygen saturation at 2 years. All patients were medically inoperable; the majority with poor respiratory function, but poor baseline PFTs did not predict pulmonary toxicity or decrease OS (27). Pre-existing interstitial lung disease (fibrosis) identified on high-resolution CT (HRCT) is, however, associated with a significantly increased risk of severe and fatal radiation pneumonitis (28) and is considered a relative contraindication to SBRT. Careful evaluation of the risks and benefits of SBRT by an expert tumor board is advised in this subgroup (7).
Chest wall toxicity
Chest wall pain, rib fractures and skin reaction are possible when treating peripheral lesions with SBRT. In their collaborative analysis of 505 patients with heterogeneous fractionation regimes, Grills et al identified rib fractures in 8% (3% grade ≥2) and grade 2 or above skin toxicity in 2%. Post-SBRT rib fractures were more common with higher BED (19). In a single institution series of 500 patients receiving risk-adapted SBRT (tumors in broad contact with the chest wall received 60 Gy/5 fractions versus 60 Gy/3 fractions for peripheral T1 tumors), chest wall pain and symptomatic rib fractures were reported in 11% and 2% respectively (29). Grade 3 chest wall toxicity occurred in 2%, associated with larger volumes of chest wall receiving 30–50 Gy and higher maximum chest wall doses. Restricting the volume of chest wall receiving ≥30 Gy to less than 30 cm3 has been recommended (30).
Comparison with other treatments
SBRT vs. 3D conformal radiotherapy (3D-CRT)
SPACE (Stereotactic Precision and Conventional radiotherapy Evaluation) is the only published randomized trial comparing SBRT (66 Gy/3 fractions over 1 week) with 3D-CRT (70 Gy/35 fractions over 7 weeks) for peripheral ES-NSCLC. There was no significant difference in OS, progression-free survival (PFS) or LC at 3 years between the two groups. HRQOL was better amongst those receiving SBRT, with decreased pneumonitis (19% vs. 34%) and esophagitis (8% vs. 30%) (31). The ability to detect a difference in outcome was hampered by the phase two design, relatively low power and imbalance of treatment arms (more patients with T2 tumors in the SBRT arm), but the authors concluded that SBRT should be regarded as standard of care due to improved convenience and HRQOL. The CHISEL study randomized 101 Australasian patients with biopsy proven inoperable peripheral ES-NSCLC to SBRT (54 Gy/3 fractions or 48 Gy/4 fractions) or 3D-CRT (66 Gy/33 fractions or 50 Gy/20 fractions). Results, presented in abstract form, suggest improved OS and freedom from local failure with SBRT (32). The OCOG-LUSTRE phase III trial is currently recruiting in Canada, comparing SBRT (48 Gy/4 fractions (peripheral lesions) or 60 Gy/8 fractions (central lesions)) to conventionally hypofractionated radiotherapy (60 Gy/15 fractions) for medically inoperable ES-NSCLC (33).
SBRT vs. surgery
Numerous studies, including population-based studies, retrospective propensity score matched comparisons and a meta-analysis, have suggested similar outcomes between surgery and SBRT (34,35). Unfortunately, all three randomized trials comparing surgery and SBRT for ES-NSCLC [STARS (NCT00840749), ROSEL (NCT00687986) and ACOSOG Z4099 (NCT01336894)] closed early because of poor accrual. Preliminary results of the STARS and ROSEL trials have been combined. In both trials surgery involved anatomic lobectomy and mediastinal lymph node dissection/sampling, SBRT regimes included 54 Gy/3 fractions (both trials), 50 Gy/4 fractions (STARS) and 60 Gy/5 fractions (ROSEL). A total of 58 patients were enrolled in both trials, with no differences in patient or tumor characteristics between the two treatment groups. At a median follow up of 40 months (SBRT) and 35 months (surgery), 3-year OS was 95% in the SBRT group and 79% in the surgical group (P=0.037) with no difference in local, regional or distant recurrence rates. Grade 3–4 treatment-related adverse events occurred in 10% with SBRT and 44% with surgery, with one death from surgical complications (36). These results have prompted several ongoing randomized trials comparing surgery and SBRT [SABRTooth (37), POSTILV (38), STABLE-MATES (39) and VALOR (40)], the long-term survival results of which are eagerly awaited. In operable patients SBRT remains controversial due to the lack of long-term survival data, omission of pathological nodal staging which may influence adjuvant therapy, difficulty interpreting post-treatment surveillance imaging and potential challenges with salvage surgery. As such, current guidelines do not recommend SBRT outside of a clinical trial for patients with standard operative risk ES-NSCLC, but shared decision-making is encouraged to define a management plan consistent with the patient’s preferences (7,41).
Central tumors
A pivotal phase II study whereby patients with ES-NSCLC received 60–66 Gy/3 fractions identified that treatment of central tumors (within 2 cm of the tracheobronchial tree) was associated with increased severe toxicity and treatment-related death (9). Patients with central tumors had a 2-year severe toxicity rate of 46% versus 17% for peripheral tumors, but OS was not statistically different (42). Reported grade 5 toxicities include hemoptysis, bronchial stricture, bronchial fistula, obstructive pneumonia and esophageal ulceration/perforation (43). As a result, SBRT regimes with ≤3 fractions are not recommended for central tumors (41). Less potent “risk-adapted” SBRT regimes over 4–8 fractions have been used in this patient group with a more favourable toxicity profile (Table 1). A systematic review of outcomes of SBRT for central lung tumors identified a risk of grade 3 or 4 toxicity of less than 9%, comparing favorably with surgery. There was an increased rate of treatment-related death with higher BED; 3.6% with BED3 ≥210 Gy (α/β =3) compared to 1% with BED3 <210 Gy. LC rates for central tumors remain above 85% with BED10 ≥100 Gy, as in peripheral tumors (43). The RTOG 0813 trial is investigating different regimes delivering 50–60 Gy in 5 fractions to central ES-NSCLC. Initial results for the higher dose arms (57.5–60 Gy/5 fractions) show 2-year LC and OS of 87–89% and 70–73%, but with grade ≥3 toxicity in 16–21% and treatment-related death in 3–5% (44). Until further studies are published, fractionation regimes close to achieving a BED10 ≥100 Gy and BED3 <210 Gy, such as 50 Gy/5 fractions or 60 Gy/8 fractions (Table 1), may provide an acceptable balance of toxicity and LC. Care should be taken to identify “ultracentral” lesions, where the tumor directly abuts major airways or the planning target volume (PTV) overlaps the trachea or main bronchi. Toxicity appears higher when treating these lesions, even using less hypofractionated schedules such as 60 Gy/12 fractions (45). SBRT is not currently recommended for “ultracentral” lesions, with more conventional or accelerated schedules being an acceptable alternative (7). A new phase I dose-escalation study, SUNSET (46), has just launched which will help address some important questions in this population. If SBRT is used for “ultracentral” lesions patients should be counselled about the potentially fatal treatment complications (41).
Other controversies, such as using SBRT for tumors greater than 5 cm, without a histological diagnosis or for multiple primary lung cancers are beyond the scope of this article, but are well summarized in the ASTRO evidence-based guideline (41).
Spinal metastases
Over 40% of all patients with cancer will develop metastatic disease to the spine. Spinal metastases are deemed “complex” bone metastases in view of their critical location which increases the risk of functional impairment. Unlike simple bone metastases, which cause pain but do not carry the same risk of neurological compromise, there are limited randomized trials available to guide management in this group. With an estimated 10% of patients with metastatic spinal disease developing spinal cord compression, an oncological emergency that may result in permanent disability, the importance of achieving LC in this group cannot be understated (47).
Although controversy remains over the optimal fractionation schedule, radiotherapy typically delivering doses of 8 Gy in a single fraction (SF) or 20–30 Gy in multiple fractions (MF) is a well-recognized modality of treatment offering effective pain control. However, the response is not durable. The Dutch Bone Metastases study comparing 8 Gy SF (n=579) with a MF regimen of 4 Gy × 6 fractions (n=578) reported a median time to response of 3 weeks in both arms with a median time to progression of 24 weeks (MF) and 20 weeks (SF), the difference in response was not significant (48). The total number of retreatments was 16%, higher in the SF arm (25% vs. 7%). Randomized trials with longer follow-up have shown progressive rates of local failure at palliative doses with re-irradiation rates of up to 42% with a SF and 24% with a MF regimen (49).
The biological effectiveness of irradiation is dependent on the total dose and dose per fraction of delivered radiation. Though useful as a palliative treatment, cEBRT is often limited by cord tolerance making dose escalation and re-irradiation difficult. SBRT is a technique whereby potentially ablative doses of radiotherapy with increased BED, can be delivered with steeper dose gradients and sub millimetre precision, minimising risk to spinal elements and other adjacent normal structures. Although there have been no randomized trials to date, results of mature single and multi-institution studies have reported better LC rates with SBRT with one prospective series of previously un-irradiated spinal disease reporting an actuarial LC rate of 88% at 18 months (50,51).
The use of survival models and frameworks to guide treatment
In the United States the incidence of spinal SBRT has increased from 2% to 20% over a 10-year period (2004–2013) (52) suggesting that since its first clinical application for spinal metastatic disease in 1995, spinal SBRT is increasing in popularity and acceptance. However, spinal SBRT in the context of metastatic disease is still a palliative treatment. Regardless of its availability, the heterogeneity of this patient population with highly variable life expectancies, the increased risk of toxicity associated with this technique and the complexity of the planning process mean careful patient selection remains paramount.
Various survival models have been developed identifying patient subgroups more likely to benefit from spinal SBRT. Once such model, the Prognostic Index for Patients with Spinal Metastases (PRISM), developed from two prospective single institution trials, suggested better survival was associated with female gender, Karnofsky performance score (KPS), previous surgery at the site of SBRT, a solitary site of disease in the spine and a disease-free interval (DFI) of >5 years. Notably pre-treatment symptom burden was significantly higher in the patient group with poor survival. The patient population was derived from prospective trials with 97% of patients exhibiting a KPS >70, so it remains to be seen whether this prognostic tool has utility when applied outside of the trial setting (53). It does however suggest that in patients with poorer performance status (PS), limited benefit is likely to be gained from spinal SBRT.
In 2013 the NOMS decision framework tried to standardise the assessment of metastatic spine tumors by incorporating neurologic, oncologic, mechanical and systemic parameters when deciding on the optimal management of spinal lesions which may include upfront surgery, cEBRT or SBRT (54). More recently, the International Spine Oncology Consortium produced two multidisciplinary algorithms for the management of spinal metastases. The framework suggests assessing the patient’s PS, systemic burden of disease and the systemic treatment options available before evaluating the spinal disease itself. The framework takes in to account mechanical stability, neurological risk (the amount of epidural disease present/cord compression), tumour histology specifically radio-sensitivity, radio-responsiveness (rapid vs. slow) and vascularity when deciding optimal management which may include SBRT, separation surgery, vertebroplasty or minimally invasive local ablative approaches (55). Such frameworks highlight the complexities involved and importance of the multidisciplinary approach when deciding on appropriate treatment in this challenging patient group.
Treatment with spinal SBRT
De novo treatment
Current evidence for management in the de novo setting (patients who have never received prior radiotherapy or surgical intervention) is derived from multiple retrospective series but few prospective trials. LC, usually defined as an absence of progression or epidural cord compression at the treated site, ranges from 68–96% at 1 year across these series (50). Although pain is the most common presenting symptom of spinal metastases, there is a paucity of studies available that include it as an end point. Limitations exist with the prospective trials reported; many include a heterogeneous study population incorporating those who had previous irradiation or surgery. Most studies have included radioresistant tumors such as melanoma, renal cell carcinoma (RCC) and sarcoma in their patient group making conclusions from subgroups analysis difficult to interpret. There is also no consensus on dose and fractionation with considerable variation throughout (50,51,56,57). Commonly used schedules include 16–24 Gy/1 fraction, 24 Gy/2 fractions, 24–27 Gy/3 fractions and 30-35 Gy/5 fractions (Table 2) (51,58-64). A multicentre randomized phase III trial comparing two dosing schedules for SBRT (27 Gy/3 fractions or 24 Gy/1 fraction) on long-term 2-year locoregional control is currently underway (65).
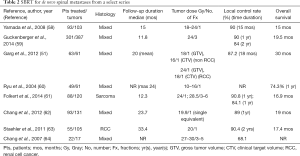
Full table
RTOG 0631, a randomized phase 3 trial comparing SBRT at 16–18 Gy/1 fraction versus cEBRT at 8 Gy/1 for pain control and HRQOL has completed accrual. The phase II component has already reported the technical feasibility and safety of delivering SF SBRT in this group (66,67). The trial will stratify between radioresistant tumors (soft tissue sarcoma, melanoma and RCC) and all other types of tumors due to expected similarity of their response to treatment, to prevent bias of the results. Previous studies have suggested no difference in LC or PFS when radioresistant tumors have been treated with SBRT. In a phase I/II trial delivering 16–24 Gy/1 fraction to 61 patients with 63 tumors, an actuarial 18-month imaging LC rate of 88% with a median survival of 30 months was recorded for all patients. Although this trial stratified according to histology [24 Gy to gross tumour volume (GTV) and 16 Gy to clinical target volume (CTV) in lesions with RCC histology versus 18 Gy to GTV and 16 Gy to CTV in non-RCC], no significant difference was noted (51). Nguyen et al. reported on 48 patients with 55 spinal metastases with RCC histology who received 24 Gy/1 fraction, 27 Gy/3 fractions or 30 Gy/5 fractions. With a median follow-up time of 13.1 months, the actuarial 1-year tumour PFS was 82.1% with 44% and 52% of patients pain free at 1 month and 12 months post-SBRT (68), comparable to other studies.
Re-irradiation
A recent systematic review of spinal re-irradiation with SBRT following cEBRT (median dose 30 Gy/10 fractions) identified only 9 single institution series (with only two prospective trials) (69-71). Overall the median 1-year LC rate was 76% (range, 66–90%) with an improvement in patients’ pain scores ranging from 65–81%. The most common adverse event was vertebral compression fracture (VCF) which developed in 12%, more common following SF treatment (though only three studies specifically reported on VCF) (69). The larger of the two prospective trials conducted at MD Anderson delivered 27 Gy/3 fractions or 30 Gy/5 fractions to 59 patients with 63 tumors (70). With a mean follow up of 17.6 months, both the 1-year radiographic LC and OS for all patients was 76%. Ninety-two percent of those treated were free from neurological deterioration from any cause at 1 year. Of the 16 tumors that progressed following re-irradiation, 13 (81.3%) were within 5 mm of the spinal cord, a group perhaps more appropriately managed with surgery considering the limitations of dose escalation while maintaining spinal cord constraints in these cases (70). There is less data regarding second salvage course SBRT following initial SBRT to the spine. Thibault et al. using a median second SBRT total dose of 30 Gy/4 fractions following a first course of SBRT with a median total dose of 24 Gy/2 fractions and cEBRT in 24 spinal segments demonstrated a LC of 81% at 1 year with median time to failure of 3 months (range, 2.7–16.7 months). Eleven of the 13 local failures were within the epidural space. This group demonstrated the feasibility and safety of salvage SBRT with no VCF or radiation myelopathy observed (72). A larger retrospective review of 162 patients (237 re-irradiation spine lesions) receiving SBRT (median dose 16 Gy/1 fraction) after at least 1 course of cEBRT or SBRT reported a VCF of 9.3% and one case of presumed radiation myelopathy (0.6%) (73).
Post-operative SBRT
A prospective randomized trial showed that decompressive surgery followed by post-operative cEBRT at a dose of 30 Gy/10 fractions is superior to radiotherapy alone with respect to ambulatory function in patients with metastatic spinal cord compression (74). There are no RCTs comparing SBRT to cEBRT in this setting however various published series currently guide management. Al-Omair et al. reported a LC rate of 84% at 1 year and 64% OS in a group of 80 patients treated with 18–26 Gy/1–2 fractions (35 patients) or 18–40 Gy/3–5 fractions (45 patients). Systemic therapy following SBRT was the only significant predictor of OS on multivariate proportional hazards analysis. Treatment with 18–26 Gy/1–2 fractions and postoperative epidural disease grade 0 (no epidural disease post-operatively) or 1 (epidural disease only compressing the dura) was a significant predictor of LC. The commonest site of failure was the epidural space likely due to under dosing of the tumour at this site to maintain spinal cord constraints, an inherent problem which can limit the efficacy of spinal SBRT (75). Separation surgery which decompresses the spinal cord by downgrading the epidural disease (74) potentially allows for ablative doses of SBRT to be delivered without risking radiation-induced myelopathy and may result in more durable LC. A retrospective review of 186 patients with epidural spinal cord compression treated with surgical decompression followed by post-operative radiation at varying dose schedules (24 Gy/1 fraction; 24–30 Gy/3 fractions or 18–36 Gy/5–6 fractions) reported a 1-year overall progression rate of 16%. The post-operative radiation dose was significantly associated with local tumour progression with a 1-year cumulative local progression rate of 4.1% for patients receiving 24–30 Gy/3 fractions, 9% for 24 Gy/1 fraction and 22.6% in 18–36 Gy/5-6 fractions (76).
Toxicity of spinal SBRT
Radiation-induced myelopathy, the most morbid complication of SBRT, is rare with a risk of <5% quoted in the literature (59,61,71,75-77). Dosimetric analysis in patients with radiation myelopathy de novo and in the re-irradiation setting has recommended safe maximum point doses to the thecal sac with a suggested minimal time to re-irradiation of at least 5 months (78,79). More commonly encountered is radiation-induced VCF. Rates of 21% following 18 Gy/1 fraction and 36–39% following 24 Gy/1 fraction suggest higher doses per fraction may result in increased risk (80) with fractionated SBRT associated with a lower overall rate of VCF. There is again a lack of randomized trials informing practice however a secondary endpoint in RTOG 0631 is to evaluate the long-term effects of SBRT on VCF and the spinal cord (66). In the acute setting, a trial using prophylactic dexamethasone (4 or 8 mg daily) resulted in a total incidence of pain flare of 19% when given 1 hour before and 4 days after SBRT, a significant decrease when compared to this group’s previously steroid naïve cohort, also treated with SBRT, where pain flare was reported at 68% (81,82).
Oligometastatic disease
Does the oligometastatic state exist?
A spectrum theory of malignancy, first hypothesized in small breast cancers, suggested that as a tumor progresses, so too does its metastatic capability. In this paradigm, tumors early in their progression, limited in number and location, could potentially be cured with loco-regional treatment. This intermediate state of metastatic progression was termed oligometastases (83,84).
Clinical evidence to support the oligometastatic state has been drawn mostly from surgical experience where aggressive resection of metastatic deposits is increasingly common with documented improvement in disease control and OS (85-89). In a surgical series of liver-limited metastatic colorectal cancer (CRC) the 5-year OS ranges from 24–58%, comparable with outcomes for stage III disease (90-93). The International Registry of Lung Metastases reporting on 5,206 cases of varying histologies following lung metastasectomy showed a 10-year OS of 34% in the best prognosis group (DFI ≥36 months and a single resectable metastases) (94). Although as early as 1982 it was suggested that surgical outcomes may be explained by a biological difference in those with isolated metastases versus widespread disease (95), recognized throughout all studies is the highly select nature of this population under investigation. In spite of an abundance of clinical evidence, there are no randomized control trials to date to support the results.
More robust data exits in patients with limited intracranial metastases. One prospective randomized trial concluded that surgical removal of a single brain metastasis followed by whole brain radiotherapy (WBRT) resulted in better LC with an improved survival from 15 to 40 weeks (P<0.01) when compared to WBRT alone (96). The RTOG 9508 phase III randomized trial reported a survival advantage in patients with a single unresectable brain metastasis receiving stereotactic radiosurgery (SRS) following WBRT versus WBRT alone of 6.5 vs. 4.9 months (P=0.0393). Although there was no survival advantage in patients with 2–3 brain metastases, SRS following WBRT improved KPS and decreased steroid use in this group (97).
There is emerging data in the literature to support SBRT as an alternative to surgery for oligometastases, offering potential treatment to those patients who are not surgical candidates. One comparative trial has shown no difference in OS in patients with pulmonary oligometastases from various biological subtypes treated with pulmonary metastasectomy (PME) or SBRT if unfit for surgery. With a median follow-up of 43 months, the OS at 1, 3 and 5 years was 87%, 62% and 41% for PME and 98%, 60% and 49% for SBRT (P=0.43) (98). It has also been suggested that consolidative radiotherapy may result in long-term disease control or even survival in patients with widespread metastatic disease who have largely responded to systemic therapy (99). A phase II randomized trial comparing local treatment (radiotherapy, including SBRT or surgery) versus maintenance therapy in patients with oligometastatic NSCLC (1–3 sites) with stable disease following first-line systemic treatment showed a PFS of 11.9 months in the consolidative group versus 3.9 months in the maintenance group (100). As a wide range of radiation doses were used in the trial (palliative doses, hypofractionation and SBRT) the question of the optimum BED for oligometastatic disease in NSCLC remains unanswered. Recently, mature results of the Surveillance or Metastasis-directed Therapy for Oligometastatic Prostate Cancer Recurrence (STOMP) trial reported. This multicentre phase II study randomized 62 patients with hormone sensitive asymptomatic biochemical recurrence of their prostate cancer (≤3 extracranial metastatic lesions) to surveillance or metastatic directed therapy (MDT) with surgery or SBRT to all detected lesions. Of the 31 patients receiving MDT, 25 received SBRT. On intent to treat analysis, the median androgen deprivation therapy (ADT)-free survival was 21 months versus 13 months in the MDT and surveillance group respectively (hazard ratio 0.60, log-rank P=0.11). An MDT directed approach was shown to be safe with no grade 2–5 adverse events and no appreciable difference in HRQOL at 1 year. Interestingly, 35% of patients in the surveillance arm experienced a spontaneous decline in prostate-specific antigen (PSA) level without treatment. This result was not durable with only 20% at 1 year and 10% at 2 years free from PSA progression suggesting that the natural progression of a small number of prostate cancers may be indolent. Further understanding of the intricate biological differences between tumors that disseminate widely and those that remain isolated with limited progression is needed to help pre-select patients suitable for MDT (101).
In the absence of evidence, who should we treat?
Several randomized trials treating oligometastatic disease with SBRT are currently underway (Table 3) (102-108) and are sure to provide valuable information on the durability of this approach across many tumour sites. The entry criteria vary with oligometastatic disease radiologically defined as anywhere between 1–5 sites. Is this an arbitrary number or a reflection of tumour burden?
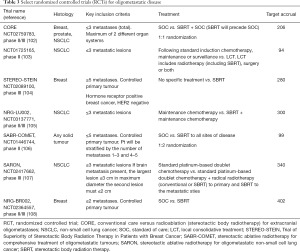
Full table
Drawing from surgical data, despite attempts to create predictive models to risk-stratify patients who may benefit from local treatment, no consensus exists within the international surgical community as to their utility or validity (109). Individual studies have suggested tumour burden is predictive of OS with the number and size of metastatic lesions (>3 hepatic metastases, hepatic metastases ≥5 cm, >1 lung metastasis), extrahepatic spread, poorly differentiated disease, positive resection margins and a short DFI (<36 months) independent predictors for poor survival (89,110). Looking at the SBRT data, Salama et al. reported a longer PFS in patients with 1–3 metastatic sites versus those with 4–5 metastases receiving escalating SBRT doses to all sites of disease (111). Multivariate analysis of the RTOG 9508 trial clearly demonstrated the best survival group was patients aged <65 with KPS ≥70, no extracranial disease and a controlled primary tumour (97).
Extrapolating from these studies, key prognostic factors have been identified to help pre-select patients for treatment (Table 4) (112). Until ongoing trials report, the oligometastatic state whereby metastatic disease can be cured with SBRT remains elusive and caution is strongly advised when selecting patients for treatment outside of a trial setting.
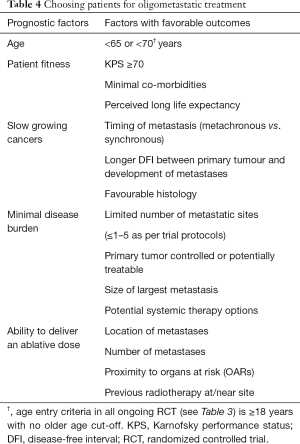
Full table
Conclusions
Recent improvements in technology and robust data have allowed the implementation of SBRT for early peripheral lung tumors with excellent results. However, the current level of evidence for SBRT in oligometastases or metastatic spinal disease is weak largely comprising multiple observational studies, pooled analyses or single arm studies without appropriate controls. Though the emerging data is compelling, it has to be balanced with the appreciable risks of SBRT in the absence of tangible survival outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Lederle FA. Lobectomy versus limited resection in T1 N0 lung cancer. Ann Thorac Surg 1996;62:1249-50. [Crossref] [PubMed]
- McGarry RC, Song G, des Rosiers P, et al. Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest 2002;121:1155-8. [Crossref] [PubMed]
- Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007;132:193-9. [Crossref] [PubMed]
- Rowell NP, Williams CJ. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable): a systematic review. Thorax 2001;56:628-38. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-21. [Crossref] [PubMed]
- Ettinger D, Wood D, Akerley W, et al. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer Version 3.2016. 2016. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Roach MC, Videtic GMM, Bradley JD, et al. Treatment of Peripheral Non-Small Cell Lung Carcinoma with Stereotactic Body Radiation Therapy. J Thorac Oncol 2015;10:1261-7. [Crossref] [PubMed]
- Chang JY, Bezjak A, Mornex F, et al. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol 2015;10:577-85. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9. [Crossref] [PubMed]
- Nguyen TK, Senan S, Bradley JD, et al. Optimal imaging surveillance after stereotactic ablative radiation therapy for early-stage non-small cell lung cancer: Findings of an International Delphi Consensus Study. Pract Radiat Oncol 2018;8:e71-8. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for Stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2011;81:e305-16. [Crossref] [PubMed]
- Chen H, Louie AV, Boldt RG, et al. Quality of Life After Stereotactic Ablative Radiotherapy for Early-Stage Lung Cancer: A Systematic Review. Clin Lung Cancer 2016;17:e141-9. [Crossref] [PubMed]
- Grills IS, Hope AJ, Guckenberger M, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol 2012;7:1382-93. [Crossref] [PubMed]
- Lagerwaard FJ, Aaronson NK, Gundy CM, et al. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol 2012;7:1148-54. [Crossref] [PubMed]
- Zhao J, Yorke ED, Li L, et al. Simple Factors Associated with Radiation-Induced Lung Toxicity After Stereotactic Body Radiation Therapy of the Thorax: A Pooled Analysis of 88 Studies. Int J Radiat Oncol Biol Phys 2016;95:1357-66. [Crossref] [PubMed]
- Giuliani M, Hope A, Guckenberger M, et al. Stereotactic Body Radiation Therapy in Octo- and Nonagenarians for the Treatment of Early-Stage Lung Cancer. Int J Radiat Oncol Biol Phys 2017;98:893-9. [Crossref] [PubMed]
- Kreinbrink P, Blumenfeld P, Tolekidis G, et al. Lung stereotactic body radiation therapy (SBRT) for early-stage non-small cell lung cancer in the very elderly (≥80years old): Extremely safe and effective. J Geriatr Oncol 2017;8:351-5. [Crossref] [PubMed]
- Cassidy RJ, Patel PR, Zhang X, et al. Stereotactic Body Radiotherapy for Early-stage Non-small-cell Lung Cancer in Patients 80 Years and Older: A Multi-center Analysis. Clin Lung Cancer 2017;18:551-8.e6. [Crossref] [PubMed]
- Hayashi S, Tanaka H, Kajiura Y, et al. Stereotactic body radiotherapy for very elderly patients (age, greater than or equal to 85 years) with stage I non-small cell lung cancer. Radiat Oncol 2014;9:138. [Crossref] [PubMed]
- Palma D, Lagerwaard F, Rodrigues G, et al. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys 2012;82:1149-56. [Crossref] [PubMed]
- Stanic S, Paulus R, Timmerman RD, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early- stage peripheral non-small cell lung cancer: an analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 2014;88:1092-9. [Crossref] [PubMed]
- Bahig H, Filion E, Vu T, et al. Severe radiation pneumonitis after lung stereotactic ablative radiation therapy in patients with interstitial lung disease. Pract Radiat Oncol 2016;6:367-74. [Crossref] [PubMed]
- Bongers EM, Haasbeek CJ, Lagerwaard FJ, et al. Incidence and risk factors for chest wall toxicity after risk-adapted stereotactic radiotherapy for early-stage lung cancer. J Thorac Oncol 2011;6:2052-7. [Crossref] [PubMed]
- Stephans KL, Djemil T, Tendulkar RD, et al. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT). Int J Radiat Oncol Biol Phys 2012;82:974-80. [Crossref] [PubMed]
- Nyman J, Hallqvist A, Lund JA, et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121:1-8. [Crossref] [PubMed]
- Ball D, Mai T, Vinod S, et al. MA 13.07 A Randomized Trial of SABR vs Conventional Radiotherapy for Inoperable Stage I Non-Small Cell Lung Cancer: TROG09.02 (CHISEL). J Thorac Oncol 2017;12:S1853. [Crossref]
- Stereotactic body radiotherapy vs conventional radiotherapy in medically-inoperable non-small cell lung cancer patients (LUSTRE). National Library of Medicine (US). 2013. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT01968941
- Louie AV, Palma DA, Dahele M, et al. Management of early-stage non-small cell lung cancer using stereotactic ablative radiotherapy: controversies, insights, and changing horizons. Radiother Oncol 2015;114:138-47. [Crossref] [PubMed]
- Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014;90:603-11. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomized trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- A Study to Determine the Feasibility and Acceptability of Conducting a Phase III Randomized Controlled Trial Comparing Stereotactic Ablative Radiotherapy (SABR) With Surgery in paTients With Peripheral Stage I nOn-small Cell Lung Cancer (NSCLC) cOnsidered Higher Risk of Complications From Surgical Resection (SABRTOOTH)). National Library of Medicine (US). 2015. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT02629458
- POSTILV: A randomized phase II trial in patients with operable stage I non-small cell lung cancer: radical resection versus ablative stereotactic radiotherapy. National Library of Medicine (US). 2012. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT01753414
- A Randomized Phase III Study of Sublobar Resection (SR) Versus Stereotactic Ablative Radiotherapy (SAbR) in High Risk Patients with Stage I Non-Small Cell Lung Cancer (NSCLC), The STABLE-MATES Trial. National Library of Medicine (US). 2015. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT02468024
- Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy Trial (VALOR). National Library of Medicine (US). 2016. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT02984761
- Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2018;36:710-9. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Senthi S, Haasbeek CJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumors: a systematic review. Radiother Oncol 2013;106:276-82. Erratum in: Radiother Oncol 2013;109:183. Dosage error in article text. [Crossref] [PubMed]
- Bezjak A, Paulus R, Gaspar LE, et al. Efficacy and Toxicity Analysis of NRG Oncology/RTOG 0813 Trial of Stereotactic Body Radiation Therapy (SBRT) for Centrally Located Non-Small Cell Lung Cancer (NSCLC). Int J Radiat Oncol Biol Phys 2016;96:S8. [Crossref]
- Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of Hypofractionated High-Dose Radiotherapy in Poor-Risk Patients with "Ultracentral" Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1081-9. [Crossref] [PubMed]
- SUNSET: SBRT for Ultra-central NSCLC- a Safety and Efficacy Trial. National Library of Medicine (US). 2017. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT03306680
- Holman PJ, Suki D, McCutcheon I, et al. Surgical management of metastatic disease of the lumbar spine: experience with 139 patients. J Neurosurg Spine 2005;2:550-63. [Crossref] [PubMed]
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101-9. [Crossref] [PubMed]
- Chow E, Hoskin PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) SC 20. Clin Oncol (R Coll Radiol) 2006;18:125-8. [Crossref] [PubMed]
- Husain ZA, Sahgal A, De Salles A, et al. Stereotactic body radiotherapy for de novo spinal metastases: systematic review. Journal of neurosurgery. Spine 2017;27:295-302. [Crossref] [PubMed]
- Garg AK, Shiu AS, Yang J, et al. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer 2012;118:5069-77. [Crossref] [PubMed]
- McClelland S 3rd, Kim E, Passias PG, et al. Spinal stereotactic body radiotherapy in the United States: A decade-long nationwide analysis of patient demographics, practice patterns, and trends over time. J Clin Neurosci 2017;46:109-12. [Crossref] [PubMed]
- Tang C, Hess K, Bishop AJ, et al. Creation of a Prognostic Index for Spine Metastasis to Stratify Survival in Patients Treated With Spinal Stereotactic Radiosurgery: Secondary Analysis of Mature Prospective Trials. Int J Radiat Oncol Biol Phys 2015;93:118-25. [Crossref] [PubMed]
- Laufer I, Rubin DG, Lis E, et al. The NOMS Framework: Approach to the Treatment of Spinal Metastatic Tumors. Oncologist 2013;18:744-51. [Crossref] [PubMed]
- Spratt DE, Beeler WH, de Moraes FY, et al. An integrated multidisciplinary algorithm for the management of spinal metastases: an International Spine Oncology Consortium report. Lancet Oncol 2017;18:e720-30. [Crossref] [PubMed]
- Bydon M, De la Garza-Ramos R, Bettagowda C, et al. The use of stereotactic radiosurgery for the treatment of spinal axis tumors: a review. Clin Neurol Neurosurg 2014;125:166-72. [Crossref] [PubMed]
- Tseng CL, Campbell M, Soliman H, et al. Imaging-Based Outcomes for 24 Gy in 2 Daily Fractions for Patients With De Novo Spinal Metastases Treated with Spine Stereotactic Body Radiation Therapy: An Emerging Standard. Int J Radiat Oncol Biol Phys 2017;99:S158. [Crossref]
- Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys 2008;71:484-90. [Crossref] [PubMed]
- Guckenberger M, Mantel F, Gerszten PC, et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: a multi-institutional analysis. Radiat Oncol 2014;9:226. [Crossref] [PubMed]
- Ryu S, Rock J, Rosenblum M, et al. Patterns of failure after single-dose radiosurgery for spinal metastasis. J Neurosurg 2004;101 Suppl 3:402-5. [PubMed]
- Folkert MR, Bilsky MH, Tom AK, et al. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys 2014;88:1085-91. [Crossref] [PubMed]
- Chang UK, Cho W, Kim M, et al. Local tumor control after retreatment of spinal metastasis using stereotactic body radiotherapy; comparison with initial treatment group. Acta Oncol 2012;51:589-95. [Crossref] [PubMed]
- Staehler M, Haseke N, Nuhn P, et al. Simultaneous anti-angiogenic therapy and single-fraction radiosurgery in clinically relevant metastases from renal cell carcinoma. BJU Int 2011;108:673-8. [PubMed]
- Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 2007;7:151-60. [Crossref] [PubMed]
- A Phase III randomized study comparing two dosing schedules for hypofractionated image guided radiation therapy in patients with metastatic cancer. National Library of Medicine (US). 2017. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/show/NCT01223248
- Radiation Therapy Oncology Group. Image guided radiosurgery or stereotactic body radiation therapy in treating patients with localized spinal metastasis (ClinicalTrials.gov NLM Identifer: NCT00922974). Bethseda. MD. National Library of Medicine 2000. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT00922974
- Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1-3) spine metastases: phase 2 results. Pract Radiat Oncol 2014;4:76. [Crossref] [PubMed]
- Nguyen QN, Shiu AS, Rhines LD, et al. Management of Spinal Metastases From Renal Cell Carcinoma Using Stereotactic Body Radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:1185-92. [Crossref] [PubMed]
- Myrehaug S, Sahgal A, Hayashi M, et al. Reirradiation spine stereotactic body radiation therapy for spinal metastases: systematic review. J Neurosurg Spine 2017;27:428-35. [Crossref] [PubMed]
- Garg AK, Wang XS, Shiu AS, et al. Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy: The University of Texas MD Anderson Cancer Center experience. Cancer 2011;117:3509-16. [Crossref] [PubMed]
- Ahmed KA, Stauder MC, Miller RC, et al. Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys 2012;82:e803-9. [Crossref] [PubMed]
- Thibault I, Campbell M, Tseng CL, et al. Salvage Stereotactic Body Radiotherapy (SBRT) Following In-Field Failure of Initial SBRT for Spinal Metastases. Int J Radiat Oncol Biol Phys 2015;93:353-60. [Crossref] [PubMed]
- Boyce-Fappiano D, Elibe E, Zhao B, et al. Reirradiation of the spine with stereotactic radiosurgery: efficacy and toxicity. Pract Radiat Oncol 2017;7:e409-17. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Al-Omair A, Masucci L, Masson-Cote L, et al. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol 2013;15:1413-9. [Crossref] [PubMed]
- Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following "separation surgery" and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine 2013;18:207-14. [Crossref] [PubMed]
- Bate BG, Khan NR, Kimball BY, et al. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J Neurosurg Spine 2015;22:409-15. [Crossref] [PubMed]
- Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys 2013;85:341. [Crossref] [PubMed]
- Sahgal A, Ma L, Weinberg V, et al. Reirradiation Human Spinal Cord Tolerance for Stereotactic Body Radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:107-16. [Crossref] [PubMed]
- Sahgal A, Whyne CM, Ma L, et al. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol 2013;14:e310-20. [Crossref] [PubMed]
- Khan L, Chiang A, Zhang L, et al. Prophylactic dexamethasone effectively reduces the incidence of pain flare following spine stereotactic body radiotherapy (SBRT): a prospective observational study. Support Care Cancer 2015;23:2937-43. [Crossref] [PubMed]
- Chiang A, Zeng L, Zhang L, et al. Pain flare is a common adverse event in steroid-naïve patients after spine stereotactic body radiation therapy: a prospective clinical trial. Int J Radiat Oncol Biol Phys 2013;86:638-42. [Crossref] [PubMed]
- Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol 1994;12:2229-34. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Bartlett EK, Simmons KD, Wachtel H, et al. The rise in metastasectomy across cancer types over the past decade. Cancer 2015;121:747-57. [Crossref] [PubMed]
- Miller G. Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg 2007;205:231-8. [Crossref] [PubMed]
- Casiraghi M, De Pas T, Maisonneuve P, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the "international registry of lung metastases". J Thorac Oncol 2011;6:1373-8. [Crossref] [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [Crossref] [PubMed]
- Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br J Cancer 2006;94:982-99. [Crossref] [PubMed]
- Primrose JN. Surgery for colorectal liver metastases. Br J Cancer 2010;102:1313-8. [Crossref] [PubMed]
- Pawlik TM, Choti M. Surgical Therapy for Colorectal Metastases to the Liver. J Gastrointest Surg 2007;11:1057-77. [Crossref] [PubMed]
- Wei AC, Greig P, Grant D, et al. Survival After Hepatic Resection for Colorectal Metastases: A 10-Year Experience. Ann Surg Oncol 2006;13:668-76. [Crossref] [PubMed]
- Morris EJA, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010;97:1110-8. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Goslin R, Steele G Jr, Zamcheck N, et al. Factors influencing survival in patients with hepatic metastases from adenocarcinoma of the colon or rectum. Dis Colon Rectum 1982;25:749-54. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A Randomized Trial of Surgery in the Treatment of Single Metastases to the Brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomized trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Widder J, Klinkenberg TJ, Ubbels JF, et al. Pulmonary oligometastases: Metastasectomy or stereotactic ablative radiotherapy? Radiother Oncol 2013;107:409-13. [Crossref] [PubMed]
- Macdermed DM, Weichselbaum RR, Salama JK. A rationale for the targeted treatment of oligometastases with radiotherapy. J Surg Oncol 2008;98:202-6. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018;36:446-53. [Crossref] [PubMed]
- Conventional Care Versus Radioablation (Stereotactic Body Radiotherapy) for Extracranial Oligometastases (CORE). National Library of Medicine (US). 2017. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT02759783
- Study Assessing the Efficacy of Local Consolidative Therapy for Non-Small Cell Lung Cancer Patients with Induced Oligometastatic Disease. National Library of Medicine (US). 2017. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT01725165
- Trial of Superiority of Stereotactic Body Radiation Therapy in Patients with Breast Cancer (STEREO-STEIN). National Library of Medicine (US). 2017. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT02089100
- Maintenance Chemotherapy with or Without Stereotactic Body Radiation Therapy in Treating Patients with Stage IV Non-Small Cell Lung Cancer. National Library of Medicine (US). 2017. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT03137771
- Stereotactic Ablative Radiotherapy for Comprehensive Treatment of Oligometastatic Tumors. (SABR-COMET) National Library of Medicine (US). 2017. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT01446744
- Stereotactic Ablative Radiotherapy for Oligometastatic Non-small Cell Lung Cancer (SARON). National Library of Medicine (US). 2017. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT02417662
- Standard of Care Therapy with or Without Stereotactic Radiosurgery and/or Surgery in Treating Patients with Limited Metastatic Breast Cancer. National Library of Medicine (US). 2017. [Internet]. 2018 Jan 22 [cited 2018 Jan 22]. Available online: https://clinicaltrials.gov/ct2/show/NCT02364557.
- Gregoire E, Hoti E, Gorden DL, et al. Utility or futility of prognostic scoring systems for colorectal liver metastases in an era of advanced multimodal therapy. Eur J Surg Oncol 2010;36:568-74. [Crossref] [PubMed]
- Rees M, Tekkis PP, Welsh FKS, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-35. [Crossref] [PubMed]
- Salama JK, Michael D, Hasselle MD, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer 2012;118:2962-70. [Crossref] [PubMed]
- Palma DA, Louie AV, Rodrigues GB, et al. New Strategies in Stereotactic Radiotherapy for Oligometastases. Clin Cancer Res 2015;21:5198-204. [Crossref] [PubMed]


