Skin cancer management—updates on Merkel cell carcinoma
Introduction
This review covers clinical experience and recent updates of skin tumors. Table 1 summarizes the currently classification of World Health Organization (WHO) for skin tumors published in 2006 (1). There are a few uncommon skin tumors in the category of neural tumors: primitive neuroectodermal tumor (PNET), Ewing sarcoma, and Merkel cell carcinoma (MCC) (2,3). The National Comprehensive Cancer Network is a useful resource for all specialties dealing with skin cancer (4).
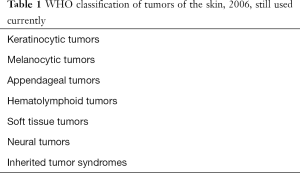
Full table
Differential diagnosis of a keratotic lesion includes keratoacanthoma, seborrhoeic keratosis, actinic keratosis, Bowen’s disease, squamous cell carcinoma, and sclerosing (morpheaform) basal cell carcinoma. A red lesion can be pyogenic granuloma, nevus, amelanotic melanoma, Kaposi’s sarcoma, lymphoma, angiosarcoma and MCC. Skin metastasis should be kept in mind.
Chemoprevention of skin cancer
Nicotinamide, an amide form of vitamin B3, has an anti-inflammatory effect such as inhibition of leukocyte chemotaxis, lysosomal enzyme release, lymphocytic transformation, and mast cell degranulation, etc. (5). Nicotinamide 500 mg BID for 12 months had been confirmed in a phase 3, double-blind randomized controlled trial of 386 cases to protect against damage caused by ultraviolet radiation (6). Within 12 months of nicotinamide, researchers found 11% decrease of new actinic keratosis, 20% decrease of new basal cell carcinoma and 30% decrease of new squamous cell carcinoma. The protective effect stopped on discontinuation of the drug. There was no adverse effect found.
Immunosuppressed patients have an increased risk of skin cancer compared to normal subjects. Nicotinamide had been tried on these patients (7). More work still has to be done for them (8). Patient should be taught self-examination of skin of the whole body. Another interesting related research on nicotinamide is the activity of two derivatives to prevent cancer metastasis in an in-vitro system (9).
Selection of optimal treatment for non-melanoma skin tumors (Tables 2,3)

Full table

Full table
Cure
Experience tells us that for small non-melanoma skin cancers, both radiation and surgery have similar cure rate. For those greater than 3 cm, we consider surgery followed by postoperative irradiation.
Cosmetic
For most skin cancers, radiation give a better cosmetic result except in the scalp where an area of alopecia is less preferred compared to a surgical scar hidden by surrounding hair. After radiotherapy, telangiectasia and changes in the background skin color can occur. For those who tend to heal with keloid, they are best served by radiotherapy than surgery.
Cost
Simple excision or biopsy by the family doctor is the least expensive treatment. Surgical removal by a plastic surgeon will have an intermediate cost. Multiple fractions of radiotherapy to a patient who has to be hospitalized would be the most expensive treatment. The elderly being confined to hospital bed with side rails up often results in muscle disuse, and pneumonia complications.
Convenience
Understandably, many patients would like to have excisional treatment by the family doctor locally. Patients do not like to be in an out of town hospital for prolonged radiotherapy. Patients with coagulopathy problems or on anticoagulant can have radiation treatment based on clinical diagnosis of skin cancer without a biopsy. Otherwise the anticoagulant has to be stopped, followed by a bridging low molecular weight heparin before biopsy and/or surgical excision.
Comfort
It depends on the site of treatment. Lesions close to the perineum, groin, oral cavity and throat are associated with severe mucositis after radiation. A small surgical scar would heal faster than the moist desquamation induced by radiation.
Choice
The choice of patients is also important. Elderly or uneducated patients are afraid of the unknown like radiation treatment. The availability of expertise also determines the final chosen treatment. An uncooperative patient is served by surgery under general anesthesia, provided consent can be obtained from family. The patient with tremor can be irradiated by wearing a radioactive mould instead of excision under local anesthesia.
Radiotherapy treatment
Table 4 summarizes commonly used regimens of external beam radiation treatments. Electronic brachytherapy is increasingly used (10). In essence, the Xoft miniature tube generates 50 kVp (kilovoltage potential) low-energy X-rays. The 4 cm applicator is placed directly over the lesion during an office procedure. The system is easy to calibrate and administer to patients (11).
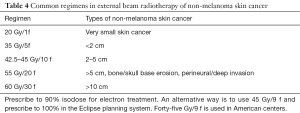
Full table
Generally skin lesions on the hand and foot are best treated by electron external beam treatment. Electronic brachytherapy can be used, as shown in a case report (12) in which each treatment only required 6 minutes. An excellent cosmetic and functional outcome was achieved at 1 year.
Systemic therapy for skin cancers
Vismodegib for basal cell carcinoma
Basal cell carcinoma is a typical example of how laboratory research can lead to advances in clinical management at bedside. Basal cell carcinoma is caused by mutations in hedgehog pathway genes. An inhibitor for this pathway, Vismodegib was found and different doses were tested in the landmark study published in 2009 (13). Vismodegib 150 mg once daily was shown to have 43% and 30% response rate in locally advanced and distant metastatic basal cell carcinomas, respectively (14). Later its use has been widened to be a neoadjuvant treatment of six months of Vismodegib prior to Mohs surgery for aggressive basal cell carcinoma (15).
When a basal cell carcinoma progresses while on Vismodegib, it can be discontinued and then restarted to induce another response (16). Researchers are studying intermittent Vismodegib therapy (17,18). Serious adverse effects of the drug occurred in 22% (108/499) patients (19). Of the 31 patients who died, 21 were the result of adverse events. Patients have an increased risk of squamous cell carcinoma after treatment (20).
Immunotherapy for melanoma
Asymptomatic brain metastases from melanoma can be treated by ipilimumab and nivolumab without local treatment according to CheckMate 204 phase II study and Australian Anti-PD1 Brain Collaboration (ABC) study (21). This is reported in American Society of Clinical Oncology (ASCO) annual meeting in 2017 and will be practice changing.
Immunotherapy for MCC
The background information for MCC is described in detail in the previous publications (22,23). Under electron microscopy, neuro-secretary granules have been found. Since MCC is a neuroendocrine carcinoma, chemotherapy regimens are similar to those for small cell lung cancer. For locally advanced or metastatic disease, cyclophosphamide/adriamycin/vincristine (CAV) had a 75.7% response rate, and etoposide/cisplatin (EP), a 55–60% response rate in a literature review (24). Toxic death from chemotherapy occurred in 3.4% in the above study. Typically the median duration of response to chemotherapy is short, 2.8 months and progression free survival is only 3.1 months. Another concern for adjuvant chemotherapy is the immunosuppressive effect which can affect the defense of the host towards MCC. At this time there is no established role of adjuvant chemotherapy in localized node negative MCC.
Newly developed immunotherapy agents include avelumab, pembrolizumab, ipilimumab and nivolumab for locally advanced or metastatic MCC. Avelumab is a human monoclonal antibody of isotype IgG1 that binds to programmed death-ligand 1 (PD-L1) and inhibits binding to receptor PD-1. It also induces antibody-dependent cell-mediated cytotoxicity. Given at a dose of 10 mg/kg every 2 weeks imparted a sustained response for previously treated metastatic MCC in phase II JAVELIN Merkel 200 trial (part A trial of 88 patients). The overall response rate was 33% (including 11.4% complete remission), lasting ≥6 or ≥12 months in 92% and 74% patients, respectively (25). In this trial, avelumab produced infusion-related reactions in 17% of patients; all were grade 1 or 2. Premedication with acetaminophen and an antihistamine is recommended prior to the first four infusions, and subsequently as needed. There were no grade 4 or 5 treatment-related adverse events. Only 4 of 88 patients (5%) had grade 3 adverse events. It had been approved by FDA in Mar 2017, irrespective of prior therapy.
The part B trial is on-going, with eligibility criteria of being first line treatment in metastatic MCC, allowing prior adjuvant treatment ≥6 m ago, immune-competent status, and Eastern Cooperative Oncology group (ECOG) performance status 0–1. A preliminary report in a poster of the ASCO in 2017 shows that it had recruited 29 patients of the 112 target (26). The overall response rate was 62.5%, and the response is still on-going so no duration of response is available yet. The response rate is higher than the above part A study among patients with previously treated metastatic MCC. There were 79.3% treatment-related adverse events but only 17.2% were grade ≥3. It is observed that current markers: PD-L1 expression, Merkel cell polyomavirus (MCPyV) status, density of CD8+ tumor-infiltrating T-cells are NOT predictive of response (27). The drug is better tolerated than chemotherapy (28).
We speculate that adjuvant systemic therapy may be indicated in pathologically node positive and recurrent cases. The exact susceptible population has not been well defined. There are two ongoing adjuvant immunotherapy trials. Professor Dirk Schadendorf, University Hospital, Essen started the “Adjuvant therapy of completely resected MCC with immune checkpoint blocking antibodies versus observation (ADMEC-O)”. It is recruiting, aiming for a target of 177 patients (29). Eligible patients are all MCC completely resected by surgery within 12 weeks before enrolment. Patients randomized to the treatment arm will receive nivolumab at a fixed dose of 480 mg by intravenous infusion every 4 weeks for up to 1 year (i.e., 13 doses). The ipilimumab arm was closed, given as a single agent (3 mg/kg) administered intravenously over a 90-minute period every 3 weeks for a total of four doses, as tolerated, i.e., day 1 (week 1), day 22 (week 4), day 43 (week 7), day 64 (week 10). The objectives are to assess overall, disease-free survival rates and adverse events.
The University of Washington started another study: “A multicenter, randomized, double-blinded, placebo-controlled, phase III trial of adjuvant avelumab (anti-PDL-1 antibody) in MCC patients with clinically detected lymph node metastases”. It is still recruiting, aiming for a target of 100 patients (30). Patients must have clinically detected nodal metastases from MCC after definitive therapy (surgery with/without adjuvant radiation therapy). They receive avelumab intravenously over 1 hour once every 15 days for the first 120 days (Induction Phase 1), once every 30 days for the next 120 days (Induction Phase 2), and then once every 120 days (Maintenance Phase) for a maximum of 720 days (approximately 24 months or 2 years total) in the absence of disease progression or unacceptable toxicity. After completion of study treatment, patients are followed up every 6 months for 3 years for a minimum of 5 years from study treatment initiation. The primary objective is relapse-free survival. Secondary objectives are to assess the overall survival, distant metastases-free survival, disease-specific survival, safety and tolerability of avelumab in the adjuvant setting.
MCC is a good example to demonstrate how laboratory researches translate to bedside clinical practice improvement. Despite many unknown factors, the current recommendation is summarized in Table 5. With effective immunotherapy available, we hope for an improved treatment outcome for MCC in the future. Similarly, more researches on other skin cancers are forth coming.
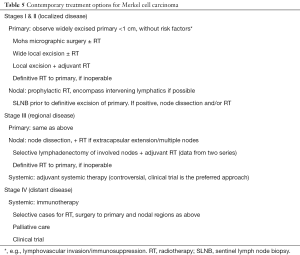
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: This article was presented in part in the 4th Hong Kong International Oncology Symposium in Hong Kong on Nov 3–4, 2017.
References
- . Available online: http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb6/Pathology and Genetics of Tumours of the Skin.
- Tai P. Pathogenesis, clinical features, and diagnosis of Merkel cell (neuroendocrine) carcinoma. Available online: www.uptodate.com
- Tai P. Staging and treatment of Merkel cell carcinoma. Available online: www.uptodate.com
- Available online: www.nccn.org
- Chen AC, Damian DL. Nicotinamide and the skin. Australas J Dermatol 2014;55:169-75. [Crossref] [PubMed]
- Chen AC, Martin AJ, Choy B, et al. A Phase 3 Randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med 2015;373:1618-26. [Crossref] [PubMed]
- Bostom AG, Merhi B, Walker J, et al. More than skin deep? Potential nicotinamide treatment applications in chronic kidney transplant recipients. World J Transplant 2016;6:658-64. [Crossref] [PubMed]
- Blomberg M, He SY, Harwood C, et al. Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol 2017;177:1225-33. [Crossref] [PubMed]
- Blazejczyk A, Switalska M, Chlopicki S, et al. 1-methylnicotinamide and its structural analog 1,4-dimethylpyridine for the prevention of cancer metastasis. J Exp Clin Cancer Res 2016;35:110. [Crossref] [PubMed]
- Ramachandran P. New era of electronic brachytherapy. World J Radiol 2017;9:148-54. [Crossref] [PubMed]
- Rong Y, Welsh JS. Surface applicator calibration and commissioning of an electronic brachytherapy system for nonmelanoma skin cancer treatment. Med Phys 2010;37:5509-17. [Crossref] [PubMed]
- Arterbery VE, Watson AC. An electronic brachytherapy technique for treating squamous cell carcinoma in situ of the digit: a case report. BMC Res Notes 2013;6:147. [Crossref] [PubMed]
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med 2009;361:1164-72. [Crossref] [PubMed]
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012;366:2171-9. [Crossref] [PubMed]
- Alcalay J, Tauber G, Fenig E, et al. Vismodegib as a neoadjuvant treatment to Mohs surgery for aggressive basal cell carcinoma. J Drugs Dermatol 2015;14:219-23. [PubMed]
- Alfieri S, Bergamini C, Granata R, et al. Retreatment with Vismodegib after progression in advanced basal cell carcinoma: first-time feport of a single-institution experience. Target Oncol 2018;13:253-6. [Crossref] [PubMed]
- Yang X, Dinehart SM. Intermittent Vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol 2016;152:223-4. [Crossref] [PubMed]
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol 2017;18:404-12. [Crossref] [PubMed]
- Basset-Seguin N, Hauschild A, Grob JJ, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol 2015;16:729-36. [Crossref] [PubMed]
- Mohan SV, Chang J, Li S, et al. Increased risk of cutaneous squamous cell carcinoma after Vismodegib therapy for basal cell carcinoma. JAMA Dermatol 2016;152:527-32. [Crossref] [PubMed]
- Combined Checkpoint Inhibition Ushers in a New Era of CNS Therapy in Melanoma. Available online: https://am.asco.org/combined-checkpoint-inhibition-ushers-new-era-cns-therapy-melanoma
- Tai P, Vu K, Torri V, et al. Merkel cell carcinoma—current state and the future. Curr Cancer Therapy Review 2016;12:79-86. [Crossref]
- Tai P. A Practical update of surgical management of Merkel cell carcinoma (MCC) of the skin. ISRN Surg 2013;2013. [Crossref] [PubMed]
- Tai PT, Yu E, Winquist E, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol 2000;18:2493-9. [Crossref] [PubMed]
- Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016;17:1374-85. [Crossref] [PubMed]
- D’Angelo S. First-line (1L) Avelumab treatment in patients (pts) with metastatic Merkel cell carcinoma (mMCC): preliminary data from an ongoing study. American Society of Clinical Oncology (ASCO) 2017:abstr 9530.
- Shapiro I. Exploratory biomarker analysis in Avelumab-treated patients with metastatic Merkel cell carcinoma progressed after chemotherapy. American Society of Clinical Oncology (ASCO) 2017:abstr 9557.
- Kaufman H. Patient experiences with Avelumab vs chemotherapy for treating Merkel cell carcinoma: results from protocol-specified qualitative research. Kaufman H. American Society of Clinical Oncology (ASCO) 2017:abstr e21065.
- . Available online: https://clinicaltrials.gov/ct2/show/NCT02196961?cond=Merkel+Cell+Carcinoma&draw=2&rank=5Adjuvant Therapy of Completely Resected Merkel Cell Carcinoma With Immune Checkpoint Blocking Antibodies Versus Observation (ADMEC-O).
- . Available online: https://clinicaltrials.gov/ct2/show/NCT03271372?cond=Merkel+Cell+Carcinoma&draw=3&rank=14Adjuvant Avelumab in Merkel Cell Cancer (ADAM).


