Neurohormonal modulation as therapeutic avenue for right ventricular dysfunction in pulmonary artery hypertension: till the dawn, waiting
Introduction
Pulmonary arterial hypertension (PAH) remains a fatal condition despite emerging and promising therapeutic options (1,2). Right ventricle (RV) function is the major determinant of prognosis in all types of PAH (3,4). As with the left ventricular dysfunction, RV dysfunction is associated with neuro-hormonal activation (5-8). The progression of RV dysfunction could be independent from that of pulmonary vascular resistances (4). Despite growing evidence supporting the role of neuro-hormonal activation in the pathogenesis of PAH related RV dysfunction, patients with PAH and RV dysfunction are not receiving neuro-hormonal modulators including beta adrenergic blockers (BB) and renin-angiotensin-aldosterone system (RAAS) inhibitors due to the fear of side effects (9).
This review highlights neuro-hormonal modulation as a prospective therapeutic avenue for the management of RV dysfunction in PAH and the need of further studies.
Why patients with PAH and RV dysfunction are not receiving neurohormonal blockade?
Evidence against the use of BB in PAH stems from experiences gathered in small PAH cohorts (10,11). Provencher et al. reported improved exercise capacity after withdrawal of propranolol which was used for prophylaxis for variceal bleeding in 10 patients with moderate-to-severe portopulmonary hypertension. An increase in heart rate after withdrawal of propranolol was thought to mediate the improvement in cardiac output and functional capacity (10). Cardiac output may largely depend on heart rate as PAH-related longstanding pressure overload steadily reduces RV myocardial contractility (9).
More recent observations indicate that patients with PAH can tolerate BB therapy. A single center experience with long term follow up (20 months) of 94 adult PAH patients (28% of patients were receiving mostly selective BBs for cardiac comorbidities) reported no detrimental effect on clinical, functional and hemodynamic outcomes including mortality (12). Tolerance to BB was confirmed over a period of 5 years by a USA-based PAH registry of 564 patients with 13% of them receiving cardio-selective BB agents (13). Lastly, Bandyopadhyay et al. showed that BB therapy was not associated with any deleterious effects for up to 78 months in PAH patients (14).
Notwithstanding their small patient populations, recent prospective studies provide proof of concept for the use of BB in PAH patients with RV dysfunction (15). A pilot study of 12 PAH patients demonstrated improvement in RV size and function after treatment with nebivolol—a third generation BB (16). Similarly, carvedilol-a third generation BB was well tolerated and improved RV function in an open label study of six type 1 PAH patients with baseline RV dysfunction (17). In a cohort of congenital heart disease patients with RV failure, Bouallal et al. demonstrated beneficial effects of BB therapy with improvement of RV ejection fraction and NYHA functional class (18). Several controlled clinical studies were undertaken to evaluate the impact of BB in PAH, however, only few patients were enrolled (Table 1). The only double blind, placebo controlled, randomized trial-PAHTCH examined the safety and benefits of carvedilol in 30 PAH patients (19). Carvedilol was well tolerated, did not affect functional capacity, and improved RV function as well as expression of beta-1 adrenergic receptor in a dose dependent manner.
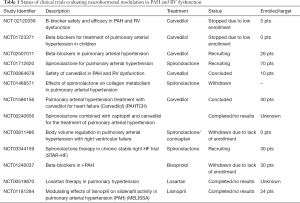
Full table
Several reasons may explain the discordant findings between older and recent studies of BB therapy in RV dysfunction: small study population and detrimental effect of propranolol on cardiac function might have contributed to the functional and hemodynamic improvement after discontinuation of propranolol (20-22). Additionally, propranolol may increase RV afterload as beta-2 adrenergic receptor blockade heightens pulmonary vascular resistance (23). The dose of metoprolol-a second generation cardioselective BB may have been excessive as noted to occur in patients with LV dysfunction who received a high initial dose of BB (21). Left ventricular systolic function declines at initiation of BB therapy in patients with heart failure with reduced ejection fraction (HFrEF). Thus one avoids initiation of BB therapy in decompensated HFrEF patients and starts at a low BB dose when have returned to a compensated state (24,25).
Experience with RAAS modulation is scarce in patients with PAH. It consists of few small uncontrolled studies that were carried out decades ago. Captopril significantly reduced pulmonary vascular resistance and pulmonary artery pressure along with improvement in RV performance independent of changes in LV systolic dysfunction (26). Improvement in RV function occurred within 4 days of captopril therapy in PAH patients (27). Although systemic arterial pressure fell, cardiac output and heart rate did not change. Stumpe et al. later on documented the sustained hemodynamic and clinical benefits of captopril in PAH patients (28). More recently, functional capacity was shown to improve after initiation of dual therapy with endothelin and mineralocorticoid receptor blockade in PAH patients (29). Use of RAAS inhibitors is not consistently effective in all PAH patients as some patients don’t derive benefits while some others may develop significant hypotension (27,30). However, the precise phenotype of such patients remains uncharacterized.
Pathophysiological basis for neurohormonal blockade in PAH with RV dysfunction
Experimental data provide clear evidence of neuro-hormonal activation and beneficial effects of neuro-hormonal modulation in PAH and RV dysfunction. Usui et al. have shown biventricular increase in local Angiotensin II, and norepinephrine reactivation of fetal gene program and hypertrophy in rats with PAH (31). Treatment with valsartan and carvedilol improved short term survival. However, due to a short follow up one does not know whether the short-term survival benefit was associated with delayed progression of PAH. Bogaard et al. provided a longer follow up and reported improved survival in rats with PAH with RV dysfunction after treatment with carvedilol. In addition, carvedilol led to improvements in exercise endurance, cardiac output and RV function (8,32). The RV functional improvement was associated with increased capillary density, lower rates of cardiomyocyte death, decreased fibrosis, and reduced pulmonary arteriolar hypertrophy and reduced pulmonary pressures. Bogaard et al. also reported the beneficial effect of beta-1 adrenergic receptor blockade with metoprolol in PAH rats. Interestingly, selective beta-1 adrenergic receptor blockade with metoprolol had a comparable effect to carvedilol, except for a lower reduction in RV hypertrophy (RVH) and dilatation; and the absence of pulmonary vascular remodeling. Thus, beta-1 adrenergic receptor blockade may prevent RVH but does not affect pulmonary vascular function. Similar findings were reported by de Man et al. using another beta-1 receptor adrenergic selective blocker-bisoprolol (33). Bunazosin hydrochloride, an alpha adrenergic blocker, may attenuate the elevation of RV systolic pressure, but not RVH in rats (34). Indeed, alpha and predominantly beta-2 receptors are found in pulmonary vasculature which might have role in PAH (23,35,36).
Overall, non-selective alpha and beta adrenergic receptor blockade may be preferred for slowing down or reversal of pulmonary vascular remodeling and prevention and progression of RV hypertrophy. The alpha and beta adrenergic receptor blocker arotinolol—an experimental drug prevented the progression of MCT-induced PAH and RVH in rat (37).
Zakheim et al. were the first to note reductions in pulmonary vascular resistance and RVH after treatment with angiotensin enzyme inhibitor (ACEI) in a hypoxia induced PAH model in rats (38). Subsequently, RAAS modulation with ACEI, angiotensin receptor blocker, mineralocorticoid receptor antagonist and ACE-2 agonist has been consistently shown to improve hemodynamics, decreased RV afterload, and reduce pulmonary vascular remodeling with arrest of pulmonary arterial smooth muscle proliferation in the absence of systemic side effects (39-46).
In summary, few clinical studies of utilizing BB and RAAS blockade/inhibition have been reported. However, a wealth of experimental data argues in favor of neuro-hormonal modulation for the treatment of PAH and RV dysfunction. Thus, human studies are needed to evaluate the role of BB and RAAS inhibition/blockade in the management of PAH with RV dysfunction.
Future directions
Even with differences between the RV and the LV including embryological origin, shape, structure, and circulatory milieu, the RV remodels similarly to the LV when subjected to increased afterload and neuro-hormonal activation (Figure 1) (47). Thus, neuro-hormonal modulation is likely to benefit patients with PAH and RV dysfunction as suggested by preclinical studies (Figure 2) (vide supra). Accordingly, a comprehensive approach to neuro-hormonal modulation akin to that used for LV dysfunction is warranted in patients with PAH and RV dysfunction. Lessons learnt from the use of pharmacological agents in HFrEF should be applied to the RV dysfunction management (19,25).

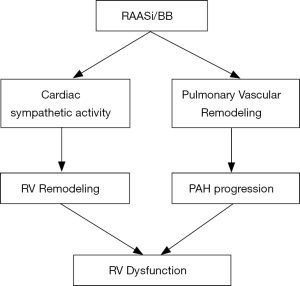
Although earlier studies utilizing non-selective first-generation BB concluded to adverse events in patients with PAH, the recent use of second and third generation BBs did not show any adverse effects and suggested positive effects on RV function and PAH progression. Definite evidence awaits controlled clinical studies. Slow enrollment has thwarted such attempts, National registries that include centers actively engaged in the management of pulmonary hypertension may uncover the benefits of BB/RAAS blockade/inhibition in PAH patients with RV dysfunction.
The preferred BB agent for PAH and RV dysfunction may be selective to pressure beta-2 adrenergic activation in the pulmonary and skeletal muscle vasculature. While metoprolol which does not affect peripheral alpha and vascular beta-2 adrenergic receptors may be better tolerated, however, carvedilol or nebivolol have vasodilatory and anti-inflammatory properties that make them attractive for the treatment of PAH with RV dysfunction. Both ACEI and ARB carry the risk of systemic arterial hypotension. However, selective angiotensin-1-receptor blockade may be more appropriate in patients with PAH and RV dysfunction who are at risk for systemic hypotension (48).
In addition to pharmacotherapy, device-based interventions directed at modulation of autonomic tone needs to be investigated. Whether or not modulation of neuro-hormonal activation shows benefits in patients with PAH and RV dysfunction, device-based modulation of autonomic tone has shown promises in the management of HFrEF (49).
Apart from heightened sympathetic activity, reduced parasympathetic activity relative to sympathetic activation contributes to morbidity and mortality, and therapeutic interventions improve clinical outcomes in HFrEF (50-52). Such approach may be desirable as reduction in sympathetic activity could be limited due to side effects of hypotension and decreased cardiac output seen with pharmacologic agents (53). Moreover, parasympathetic activation might provide additional myocardial benefits when added to the background sympathetic nervous system (SNS) and RAAS modulators (54). Restoration or augmentation of vagal tone can be achieved by electrical stimulation of vagal nerve, pharmacological approach, and exercise strategies.
Moreover, in addition to the management of established RV dysfunction in PAH, the neuro-hormonal modulation may be more effective in early PAH stages for prevention of RV dysfunction as SNS and RAAS activation are involved early in the pathogenesis of RV dysfunction (Figure 3).
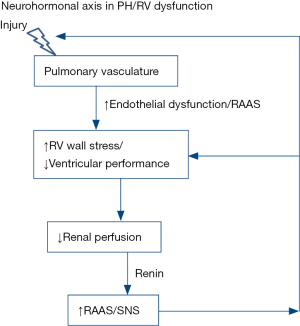
Conclusions
Neuro-hormonal activation is common in PAH and RV dysfunction. Whether this activation is the cause or the result of RV dysfunction remains uncertain. Experimental models support neuro-hormonal modulation in pressure overload RV failure. However, clinical experience is inconclusive and suffers from methodological limitations. Neuro-hormonal modulation has evolved from being contraindicated to now being the back bone of HFrEF management. One needs to systematically evaluate the clinical impact of neurohormonal modulation in patients with PAH and RV dysfunction.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156-63. [Crossref] [PubMed]
- McLaughlin VV, Hoeper MM, Channick RN, et al. Pulmonary Arterial Hypertension-Related Morbidity Is Prognostic for Mortality. J Am Coll Cardiol 2018;71:752-63. [Crossref] [PubMed]
- McLaughlin VV, Shah SJ, Souza R, et al. Management of pulmonary arterial hypertension. J Am Coll Cardiol 2015;65:1976-97. [Crossref] [PubMed]
- van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011;58:2511-9. [Crossref] [PubMed]
- Velez-Roa S, Ciarka A, Najem B, et al. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004;110:1308-12. [Crossref] [PubMed]
- Bristow MR, Minobe W, Rasmussen R, et al. Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J Clin Invest 1992;89:803-15. [Crossref] [PubMed]
- Mak S, Witte KK, Al-Hesayen A, et al. Cardiac sympathetic activation in patients with pulmonary arterial hypertension. Am J Physiol Regul Integr Comp Physiol 2012;302:R1153-7. [Crossref] [PubMed]
- Ciarka A, Doan V, Velez-Roa S, et al. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med 2010;181:1269-75. [Crossref] [PubMed]
- Holverda S, Gan CT, Marcus JT, et al. Impaired stroke volume response to exercise in pulmonary arterial hypertension. J Am Coll Cardiol 2006;47:1732-3. [Crossref] [PubMed]
- Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology 2006;130:120-6. [Crossref] [PubMed]
- Peacock A, Ross K. Pulmonary hypertension: a contraindication to the use of {beta}-adrenoceptor blocking agents. Thorax 2010;65:454-5. [Crossref] [PubMed]
- So PP, Davies RA, Chandy G, et al. Usefulness of beta-blocker therapy and outcomes in patients with pulmonary arterial hypertension. Am J Cardiol 2012;109:1504-9. [Crossref] [PubMed]
- Thenappan T, Roy SS, Duval S, et al. beta-blocker therapy is not associated with adverse outcomes in patients with pulmonary arterial hypertension: a propensity score analysis. Circ Heart Fail 2014;7:903-10. [Crossref] [PubMed]
- Bandyopadhyay D, Bajaj NS, Zein J, et al. Outcomes of beta-blocker use in pulmonary arterial hypertension: a propensity-matched analysis. Eur Respir J 2015;46:750-60. [Crossref] [PubMed]
- Moretti C, Grosso Marra W, D'Ascenzo F, et al. Beta blocker for patients with pulmonary arterial hypertension: A single center experience. Int J Cardiol 2015;184:528-32. [Crossref] [PubMed]
- Martyniuk TV, Konosova ID, Chazova IE. Use of nebivolol in patients with idiopathic pulmonary hypertension: results of the pilot study. Ter Arkh 2012;84:49-53. [PubMed]
- Grinnan D, Bogaard HJ, Grizzard J, et al. Treatment of group I pulmonary arterial hypertension with carvedilol is safe. Am J Respir Crit Care Med 2014;189:1562-4. [Crossref] [PubMed]
- Bouallal R, Godart F, Francart C, et al. Interest of beta-blockers in patients with right ventricular systemic dysfunction. Cardiol Young 2010;20:615-9. [Crossref] [PubMed]
- Farha S, Saygin D, Park MM, et al. Pulmonary arterial hypertension treatment with carvedilol for heart failure: a randomized controlled trial. JCI Insight 2017.2. [Epub ahead of print]. [PubMed]
- Wendt T, van der Does R, Schrader R, et al. Acute hemodynamic effects of the vasodilating and beta-blocking agent carvedilol in comparison to propranolol. J Cardiovasc Pharmacol 1987;10 Suppl 11:S147-50. [Crossref] [PubMed]
- Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation 2000;101:558-69. [Crossref] [PubMed]
- Malenfant S, Perros F. beta-blockers in pulmonary arterial hypertension: generation might matter. Eur Respir J 2016;47:682-4. [Crossref] [PubMed]
- Leblais V, Delannoy E, Fresquet F, et al. beta-adrenergic relaxation in pulmonary arteries: preservation of the endothelial nitric oxide-dependent beta2 component in pulmonary hypertension. Cardiovasc Res 2008;77:202-10. [Crossref] [PubMed]
- Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:e391-479. [Crossref] [PubMed]
- Bristow MR. Treatment of chronic heart failure with beta-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res 2011;109:1176-94. [Crossref] [PubMed]
- Niarchos AP, Roberts AJ, Laragh JH. Effects of the converting enzyme inhibitor (SQ 20881) on the pulmonary circulation in man. Am J Med 1979;67:785-91. [Crossref] [PubMed]
- Ikram H, Maslowski AH, Nicholls MG, et al. Haemodynamic and hormonal effects of captopril in primary pulmonary hypertension. Br Heart J 1982;48:541-5. [Crossref] [PubMed]
- Stumpe KO, Schmengler K, Bette L, et al. Persistent hemodynamic and clinical improvement after captopril in patients with pulmonary hypertension. Herz 1986;11:217-25. [PubMed]
- Maron BA, Waxman AB, Opotowsky AR, et al. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] study 1 and 2 trials). Am J Cardiol 2013;112:720-5. [Crossref] [PubMed]
- Leier CV, Bambach D, Nelson S, et al. Captopril in primary pulmonary hypertension. Circulation 1983;67:155-61. [Crossref] [PubMed]
- Usui S, Yao A, Hatano M, et al. Upregulated neurohumoral factors are associated with left ventricular remodeling and poor prognosis in rats with monocrotaline-induced pulmonary arterial hypertension. Circ J 2006;70:1208-15. [Crossref] [PubMed]
- Bogaard HJ, Natarajan R, Mizuno S, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med 2010;182:652-60. [Crossref] [PubMed]
- de Man FS, Handoko ML, van Ballegoij JJ, et al. Bisoprolol delays progression towards right heart failure in experimental pulmonary hypertension. Circ Heart Fail 2012;5:97-105. [Crossref] [PubMed]
- Inoue M, Watanabe K, Mori C, et al. The effect of bunazosin on monocrotaline-induced pulmonary hypertension in rats. Acta Paediatr Jpn 1994;36:133-8. [Crossref] [PubMed]
- Salvi SS. Alpha1-adrenergic hypothesis for pulmonary hypertension. Chest 1999;115:1708-19. [Crossref] [PubMed]
- Colucci WS, Holman BL, Wynne J, et al. Improved right ventricular function and reduced pulmonary vascular resistance during prazosin therapy of congestive heart failure. Am J Med 1981;71:75-80. [Crossref] [PubMed]
- Ishikawa M, Sato N, Asai K, et al. Effects of a pure alpha/beta-adrenergic receptor blocker on monocrotaline-induced pulmonary arterial hypertension with right ventricular hypertrophy in rats. Circ J 2009;73:2337-41. [Crossref] [PubMed]
- Zakheim RM, Mattioli L, Molteni A, et al. Prevention of pulmonary vascular changes of chronic alveolar hypoxia by inhibition of angiotensin I-converting enzyme in the rat. Lab Invest 1975;33:57-61. [PubMed]
- de Man FS, Tu L, Handoko ML, et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;186:780-9. [Crossref] [PubMed]
- Maron BA, Zhang YY, White K, et al. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation 2012;126:963-74. [Crossref] [PubMed]
- Rouleau JL, Kapuku G, Pelletier S, et al. Cardioprotective effects of ramipril and losartan in right ventricular pressure overload in the rabbit: importance of kinins and influence on angiotensin II type 1 receptor signaling pathway. Circulation 2001;104:939-44. [Crossref] [PubMed]
- Rondelet B, Kerbaul F, Van Beneden R, et al. Prevention of pulmonary vascular remodeling and of decreased BMPR-2 expression by losartan therapy in shunt-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 2005;289:H2319-24. [Crossref] [PubMed]
- Okada M, Harada T, Kikuzuki R, et al. Effects of telmisartan on right ventricular remodeling induced by monocrotaline in rats. J Pharmacol Sci 2009;111:193-200. [Crossref] [PubMed]
- Yamazato Y, Ferreira AJ, Hong KH, et al. Prevention of pulmonary hypertension by Angiotensin-converting enzyme 2 gene transfer. Hypertension 2009;54:365-71. [Crossref] [PubMed]
- Johnson JA, West J, Maynard KB, et al. ACE2 improves right ventricular function in a pressure overload model. PLoS One 2011;6. [Crossref] [PubMed]
- Preston IR, Sagliani KD, Warburton RR, et al. Mineralocorticoid receptor antagonism attenuates experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2013;304:L678-88. [Crossref] [PubMed]
- Bristow MR, Quaife RA. The adrenergic system in pulmonary arterial hypertension: bench to bedside (2013 Grover Conference series). Pulm Circ 2015;5:415-23. [Crossref] [PubMed]
- de Man FS, Handoko ML, Guignabert C, et al. Neurohormonal axis in patients with pulmonary arterial hypertension: friend or foe? Am J Respir Crit Care Med 2013;187:14-9. [Crossref] [PubMed]
- Singh JP, Kandala J, Camm AJ. Non-pharmacological modulation of the autonomic tone to treat heart failure. Eur Heart J 2014;35:77-85. [Crossref] [PubMed]
- Olshansky B, Sabbah HN, Hauptman PJ, et al. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation 2008;118:863-71. [Crossref] [PubMed]
- Patel HC, Rosen SD, Lindsay A, et al. Targeting the autonomic nervous system: measuring autonomic function and novel devices for heart failure management. Int J Cardiol 2013;170:107-17. [Crossref] [PubMed]
- Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev 2011;16:101-7. [Crossref] [PubMed]
- Da Silva Goncalves Bos D, Sun X, Vonk-Noordegraaf A, et al. Parasympathetic Nervous System Stimulation by Pyridostigmine Improves Survival and Cardiac Function in Experimental Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2016;193:A3878.
- Li M, Zheng C, Kawada T, et al. Adding the acetylcholinesterase inhibitor, donepezil, to losartan treatment markedly improves long-term survival in rats with chronic heart failure. Eur J Heart Fail 2014;16:1056-65. [Crossref] [PubMed]


