Management of hormone-receptor positive human epidermal receptor 2 negative advanced or metastatic breast cancers
Introduction
Except for a minority of patients, most patients with metastatic breast cancer are incurable. Treatments for such patients aim at controlling symptoms, maintaining the quality of life, and preventing serious complications arising from the cancer progression, with a view to delaying the time to cancer progression and if possible prolonging their overall survival (OS). Hormonal therapy is the mainstay treatment for those patients with hormone-receptor positive (HR+) human epidermal receptor 2 negative (HER2−) advanced or metastatic breast cancer (A/MBC), which should prevail in later lines of therapy until hormonal resistance is observed (1-3). On the other hand, cytotoxic chemotherapy should be indicated in those patients presenting with visceral crisis and/or hormone resistance (1-3). As defined in the Advanced Breast Cancer 2 (ABC2) guideline, cancer relapses observed 1 year or longer after completion of adjuvant hormone treatment are considered hormone-sensitive (4). Relapses occurring during the 1st 2 years of adjuvant hormone therapy or within the first 6 months of hormone therapy for A/MBC are regarded as having primary hormone-resistant diseases, and relapses from year 3 to year 6 after initiation of adjuvant hormone therapy or more than 6 months form commencement of hormone therapy for A/MBC are having secondary hormone-resistant diseases (4).
New guidelines published in 2016 by both American Society of Clinical Oncology (ASCO) and European Society of Medical Oncology (ESMO) specifically for HR+ A/MBC have incorporated the various new drugs recently added to the armamentarium of targeted therapies (1,2). In this review article, various options of first-line (1L) therapy for HR+ HER2− A/MBC will be reviewed, making specific reference to the latest evidence in medical literature reported in recent years.
Chemotherapy
Single agent chemotherapy was compared with hormone therapy in a Cochrane database systemic review published in 2003 (5). Although response rate to chemotherapy was higher than hormone therapy, there was no significant benefit in OS reported in six randomized studies reviewed (5). The review concluded that standard 1L treatment for patients with A/MBC should be hormone therapy rather than chemotherapy, except for rapidly progressing disease (5). Both the ESMO and ASCO guidelines considered hormone therapy the preferred treatment for HR+ HER2− A/MBC even in the presence of visceral metastasis unless there is visceral crises or endocrine resistance (1,2).
Single hormone therapy
Selective estrogen receptor modulator (SERM) such as tamoxifen competes with endogenous estrogen for estrogen receptors (ER), interfering with mitogenic signal transduction and halting tumor proliferation (6). Aromatase inhibitor (AI) inhibits aromatase-dependent estrogen synthesis in the peripheral and breast tissues in estrogen-deprived environment of post-menopausal women and therefore also interferes with the estrogen-dependent tumor growth (6). Multiple randomized studies have shown various AIs of anastrozole, letrozole, and exemestane produced superior outcomes compared with tamoxifen when used as 1L hormone therapy (7-10). Compared to tamoxifen, AI produced an additional 10% overall response rate (ORR), an additional 10% clinical benefit rate (CBR), and an additional 4 months median progression-free survival (PFS) (7-10).
On the other hand, selective estrogen receptor down-regulator (SERD) degrades and reduces ER and retards cell growth (6). The FALCON study randomized 450 post-menopausal patients with A/MBC who had not received any hormone therapy, to either an AI (anastrozole) or SERD (fulvestrant at 500 mg) (11). Fulvestrant improved PFS, the primary endpoint, when compared with anastrozole in patients without visceral metastasis [22.3 vs. 13.8 months; hazard ratio (HR) =0.59, 95% CI: 0.42–0.84], but there was no difference in PFS between the 2 treatment arms in those with visceral metastasis (13.8 vs. 15.9 months; HR =0.99, 95% CI: 0.74–1.33) (11). Follow-up period was still too short for assessing the secondary endpoint of OS. Based on this study, fulvestrant may be the drug of choice as 1L hormone monotherapy in patients with no visceral metastasis.
Combination hormone therapy
Given the potential synergism in controlling tumor proliferation with concurrent use of both SERD and AI, the combination was investigated in 2 randomized studies both published in 2012 (12,13). The SWOG S0226 study randomized 694 patients to receive either anastrozole alone, or anastrozole plus fulvestrant (250 mg) (12). Prior adjuvant AI was allowed if relapse or metastasis occurred at least 1 year after completion of adjuvant AI, but patients included should not have received prior hormone therapy for A/MBC. Median PFS (15 vs. 13.5 months, P=0.007; HR =0.80, 95% CI: 0.68–0.94) and median OS (47.7 vs. 41.3 months, P=0.049; HR =0.81, 95% CI: 0.65–1.0) were improved by 1.5 and 6 months respectively, when fulvestrant was added to anastrozole (12).
Another study with similar design reported different outcomes. Combination fulvestrant and anastrozole did not confer benefit in either median time to progression (10.8 vs. 10.2 months) or OS (37.8 vs. 38.2 months) in the FACT study (13). In the SWOG study, 40% patients presented with de novo metastatic disease and hence were hormone-naïve, and 60% patients had received no adjuvant tamoxifen or AI, whereas 70% patients in the FACT study had received prior anti-estrogen (12,13). The studies suggested fulvestrant worked best when used early before exposure to AI or any hormone therapy. Despite the conflicting results of the 2 studies, combination fulvestrant and AI is considered a viable 1L option in A/MBC in both ASCO and ESMO guidelines, particularly for those patients without prior exposure to hormone therapy (1,2).
It is interesting to note that clinical progression of breast cancer did occur rather early after commencement of hormone monotherapy by either AI or fulvestrant, with the PFS Kaplan-Meier curves beginning to drop in the first few months (12,13). This observation indicates that there is room for improving outcome right from the early phase of hormone-based treatment by novel therapies.
Combination hormone therapy and targeted therapy
Basic cellular and translational research have identified multiple molecular signaling pathways that are important in HR+ breast cancers, including the phosphatidylinositol 3-kinase (PI3K) pathway, mammalian target of rapamycin (mTOR) pathways, and the cyclin-dependent kinase (CDK) dependent cell cycle pathway (14,15). However, overexpression of these pathways may be variable in different phases along the clinical course of HR+ cancers, which can be altered by exposure to hormone therapies (14,15).
mTOR inhibitors
An inhibitor of mTOR pathway, temsirolimus, was studied in a randomized phase III placebo-controlled study as 1L endocrine therapy. In the HORIZON study, 1,106 patients were randomized to AI alone or AI plus temsirolimus (16). Patients with A/MBC could be eligible if they had not received prior hormone therapy for A/MBC, and if the metastasis was detected at least 6 months from completion of adjuvant hormone therapy with no adjuvant AI within the past 12 months. Unfortunately, temsirolimus did not confer benefit in PFS or OS over placebo when added to AI (16).
Another mTOR inhibitor, everolimus, was added to Letrozole in a phase 2 single arm study in HR+ HER2− A/MBC (BOLERO-4) (17). Eligible hormone-sensitive patients (defined by ABC2) received the combination everolimus and non-steroidal AI (NSAI) given as 1L hormone therapy. The design of the study allowed continuation of everolimus to be combined with a steroidal AI (exemestane) upon disease progression. At a median follow-up period of 29.5 months, the median PFS was 22.0 months, and median OS had not been reached (17). The ORR and CBR was 42.6% and 74.3% respectively (17). The study concluded that combination everolimus and letrozole is an effective 1L regimen for HR+ HER2− A/MBC. Only a small proportion of patients (25%) had continued everolimus in combination with exemestane as 2L therapy which showed limited efficacy with a short median PFS of 3.7 months (17).
CDK 4/6 inhibitors
Growth of HR+ breast cancer is found to be dependent on cyclin D1 (encoded by CCND1) which is a direct transcriptional target of ER. Cyclin D1 activates Cyclin-dependent kinases 4 and 6 (CDK4/6), and the interaction of cyclin D with CDK4/6 facilitates the hyperphosphorylation of the retinoblastoma (Rb) gene product, leading to cell cycle transition from G1 to S phase cell cycle (18,19). Palbociclib, ribociclib and abemaciclib are three orally bioavailable, selective, small-molecule CDK4/6 inhibitors (CDK4/6i) that block the phosphorylation of retinoblastoma protein, thereby preventing cell-cycle progression and inducing G1 phase arrest (18,19). The 3 CDK4/6i have some differences in IC50s for different CDKs and hence variations in the pattern of toxicity were observed in clinical studies testing different CDK4/6i (18). Over the past 3 to 4 years, multiple randomized phase 2 or 3 studies on using CDK4/6i in managing HR+ HER2− MBC as first, second, or more lines of therapy have been reported, providing level 1 evidence benefits of CDK4/6i in 1L treatment of HR+ HER2− breast cancers (20-27). Results of these studies are summarized in Table 1.
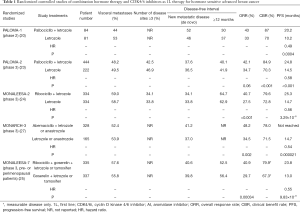
Full table
Palbociclib was combined with letrozole in a randomized phase II study (PALOMA-1) (20). A total of 165 patients with HR+ HER2− hormone-sensitive A/MBC were randomized to receive Letrozole alone, or combination Letrozole plus palbociclib (125 mg QD, days 1–21 in 28-day cycles) as 1L therapy (20). Palbociclib-treated patients had an ORR of 43% and a median PFS of 20.2 months, which were statistically superior to the ORR of 33% and median PFS of 10.2 months respectively for patients treated by letrozole alone (20). Consistent superior outcomes in terms of ORR and PFS were confirmed in 666 post-menopausal patients recruited in a subsequent randomized phase III study (PALOMA-2) (23). All patients had not received prior hormone therapy for advanced disease, and/or relapsed at least 12 months or more from adjuvant AI. ORR of 42% and median PFS of 24.8 months were reported in patients treated by combination palbociclib and letrozole, which were significantly better than the ORR of 35% and median PFS of 14.5 months reported in patients treated by letrozole alone (23). All subgroups benefited from the combination therapy in subgroup analysis (23).
Ribociclib (600 mg QD, days 1–21 of 28-day cycle) added to letrozole was compared with letrozole alone in 668 patients enrolled in the MONALEESA II study (24). Patients treated by combination therapy had statistically superior ORR (42.5% vs. 28.7%), and statistically longer median PFS (25.3 vs. 14.7 months, P=0.0003; HR =0.56; 95% CI: 0.43–0.72) (24). Subgroup analysis showed consistent PFS benefit in all subgroups (24). Recently, preliminary results of MONALEESA-7 study targeting pre or peri-menopausal patients were reported (25). Eligible patients with hormone-sensitive A/MBC received ovarian suppression by goserelin plus either tamoxifen or NSAI, and either ribociclib or placebo by randomization as 1L hormone therapy (25). PFS was significantly improved in the ribociclib arm (23.8 months) compared with the placebo arm (13.0 months), while the ORR was also significantly higher among patients with measurable disease in the ribociclib arm compared with the placebo arm (51% vs. 36%) (25).
The MONARCH 3 study had similar design to both PALOMA-2 and MONALEESA-2 studies (27). Abemaciclib (150 mg Bid, continuous schedule) was added to AI as combination therapy which was compared with AI alone in the randomized phase III study, in which 493 HR+ HER2− hormone-sensitive patients with A/MBC were randomized in a 1:1 ratio (27). Similar to the previous 2 studies, both ORR and median PFS were superior in the combination arm: ORR was 48% (vs. 35%) and median PFS had not been reached (vs. 14.7 months, P=0.000021; HR =0.54; 95% CI: 0.41–0.72) (27).
With ORR and PFS benefits demonstrated in 5 randomized studies (4 phase III and 1 phase II) (20,23-25,27), combination of CDK4/6i (palbociclib and ribociclib) with AI have been approved by FDA as 1L therapy for HR+ HER2− A/MBC. This has been incorporated in the ESO-ESMO ABC3, ASCO and National Comprehensive Cancer Network (NCCN) guidelines (1-3). Apart from 1L therapy, combination of various CDK4/6i with fulvestrant have also been approved by FDA or EMA as 2L or later lines of therapy in patients previously treated with hormone therapy. Interestingly, both the ORR and the median PFS reported in patients treated by AI alone in the control arms were extremely consistent across the 3 1L studies, ranging from 28–35% for ORR and 14.5–14.7 months for median PFS, indicating the rather uniform patient inclusion criteria in the 3 randomized studies (23,24,27) (Table 1). In contrast to the early drop of PFS alluded to earlier in hormone monotherapy, the CDK4/6i combination conferred an improvement in PFS shortly after commencement of treatment, exemplified by the early separation of the Kaplan-Meier PFS curve of CDK4/6i combination arm from the AI alone arm (20,23-25,27).
2L therapy after 1L CDK4/6i
With incorporation of novel targeted therapies into the 1L treatment algorithm for HR+ HER2− advanced breast cancer, the optimal 2L therapy for patients who have failed 1L CDK4/6i remains to be defined. Despite conferment of an additional 10 months of median PFS, most patients treated by upfront CDK4/6i and AI will still develop hormone resistance with time and progress (20,23-25,27), potentially calling for an effective 2L hormone-based treatment if the patient is not indicated for chemotherapy. Based on the interplay of various molecular pathways identified in HR+ HER− breast cancers (6,14,15,18,19), potential options for circumventing hormone resistance are summarized below (28-32) (Table 2):
- Tamoxifen + everolimus (TAMRAD study) (28);
- Exemestane + everolimus (BOLERO-2 study) (29,30);
- Fulvestrant + buparlisib (pan-PI3k inhibitor) (BELLE-2 study) (31);
- Fulvestrant + palbociclib (PALOMA-3 study) (22)/abemaciclib (MONARCH-2 study) (26);
- Clinical trial or genomic test to guide treatment.
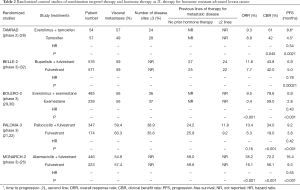
Full table
Combination hormone therapy with mTOR inhibitors
In a randomized phase II study conducted by the GINECO group, 111 patients with prior exposure to hormone treatment received tamoxifen (20 mg/day) alone, or tamoxifen (20 mg/day) with everolimus (10 mg/day) (28). Addition of everolimus improved both time to progression (8.6 vs. 4.5 months, P=0.0021; HR =0.45, 95% CI: 0.26–0.81) for the whole group (28). The 6-month CBR was 61% (95% CI: 47–74%) with tamoxifen plus everolimus, and was 42% (95% CI: 29–56%) with tamoxifen alone (28). Risk of death was reduced by 55% with tamoxifen plus everolimus versus tamoxifen alone (HR =0.45; 95% CI: 0.24–0.81) (28). Subgroup analysis based on type of hormone resistance (primary resistance defined as relapse <6 months from adjuvant AI or progression <6 months of AI therapy for metastasis) showed the combination therapy could only benefit those with secondary resistance (28).
In the BOLERO-2 study, everolimus combined with a steroidal AI, exemestane, was compared with exemestane alone (29). A total of 724 patients who had failed NSAI (anastrozole or letrozole) were randomized in a 2:1 ratio to the combination therapy and exemestane alone respectively (29). All such “hormone-resistant” patients should have had progression during or within 12 months of adjuvant NSAI, or during or less than 1 month from the end of 1L NSAI for MBC. In BOLERO-2 there was a statistically significant and clinically relevant improvement in PFS for the combination (median 6.9 vs. 2.8 months, P<0.0001; HR =0.43, 95% CI: 0.35–0.54) (29). Patients with or without visceral metastases did not apparently differ in degree of benefit in PFS conferred by combination everolimus and exemestane (29). There was also a statistically significant improvement in ORR (0.4% in the exemestane alone group vs. 9.5% in the everolimus plus exemestane group, P<0.001) (29). A recent update of BOLERO-2 reported a non–statistically significant difference in OS in favour of the combination (OS, 31.0 vs. 26.6 months, P=0.14; HR =0.89, 95% CI: 0.73–1.10) (30). The causes for the lack of OS difference may be multiple, including the subsequent lines of systemic therapy they received after failing the study treatment.
Combination hormone therapy with PI3k inhibitor
Hormone resistance and disease progression could be associated with activating PI3K mutations in HR+ breast cancer previously treated with hormone therapy (6,14,15). Combination buparlisib (a pan-PI3K inhibitor) and fulvestrant was compared with fulvestrant alone in the BELLE-2 study, a randomized double-blind placebo-controlled phase III study in postmenopausal HR+ HER2− advanced breast cancer (31). Patients who had hormone- resistant tumors defined similar to the BOLERO-2 study were eligible. Among the 1,147 patients randomized, those receiving combination treatment had better median PFS of 6.9 months compared with 5.0 months in those receiving fulvestrant alone (31). In a subgroup of 372 patients tested for presence of activated PI3K mutation in the tumor tissue, the difference in median PFS in favour of PI3K inhibition was even bigger, 6.8 vs. 4.0 months (31).
After a protocol amendment to allow mandatory blood collection, assay for ctDNA for PIK3CA mutation was possible in 587 patients (31). PI3K inhibition was able to significantly prolong PFS (7 vs. 3.2 months) in those with detectable ctDNA PIK3CA mutant (n=200), whereas PI3K inhibition did not bring significant PFS benefit to those with no detectable ctDNA mutant (n=387) (31). The study concluded that PI3K inhibition in combination with NSAI provides clinically meaningful PFS benefit in post-menopausal HR-resistant advanced BC harbouring ctDNA PIK3CA mutations in the exploratory analysis, hypothesising ctDNA assay in liquid biopsy may help select appropriate patients (31). However, significant side effects of buparlisib call for PI3Ka-specific inhibitors to be combined with hormones to offer better therapeutic ratio (31).
Combination hormone therapy with CDK4/6i
PALOMA-3 study compared fulvestrant and fulvestrant with palbociclib as 2L therapy for hormone-treated advanced breast cancer (21,22). A total of 521 patients with HR+ HER2− breast cancers who had either progressed during or within 12 months of adjuvant hormone therapy, or progressed while on hormone therapy for metastatic or recurrent cancer, were randomized in a 2:1 ratio to receive palbociclib + fulvestrant, or fulvestrant alone until disease progression. Up to 60% had visceral metastasis. Combination palbociclib and fulvestrant produced more than 5 months’ gain over fulvestrant alone in median PFS (9.2 vs. 3.8 months, P<0.001; HR =0.42, 95% CI: 0.32–0.56) (21,22).
The second CDK4/6i, abemaciclib, was investigated as 2L or more line of hormone-based therapy in the MONARCH-2 study (26). Similar patients with hormone resistance were recruited and randomized in a 2:1 ratio to receive combination abemaciclib and fulvestrant, or fulvestrant alone. Median PFS of the abemaciclib plus fulvestrant arm was 16.4 months, compared with 9.3 months in the control arm (P<0.001; HR =0.553: 95% CI: 0.45–0.68) (26). Various subgroups categorized by various parameters, such as ethnicity, age, menopausal status, number and site of metastases experienced benefit from abemaciclib to the same extent without significant difference (26). The potential benefit of ribociclib plus fulvestrant is being investigated in the randomized phase III MONALEESA-3 study (NCT02422615) which has already completed patient accrual with results to be expected soon.
Genomic tests to guide therapy
According to the recommendation of the ASCO guideline (2), it is not advisable to customize the treatment, whether hormone-based or not, on the genomic or expression profiling of the tumour. The intrinsic genomic types have been shown to portend prognosis (32), but not to aid in the selection of effective treatment for metastatic or recurrent breast cancer.
Toxicities of new targeted therapeutic drugs
Significantly higher rates of fatigue and grade 3 stomatitis were reported in patients treated with everolimus compared with placebo in the Bolero-2 study, which were associated with higher drug discontinuation rate; other potentially serious complications such as pneumonitis and hyperglycaemia were relatively uncommon (29). On the other hand, there were significantly higher rates of grade 3 to 4 neutropenia, without an increase in febrile neutropenia, in patients receiving CDK4/6i, with higher incidence (>50%) reported after palbociclib and ribociclib, than in abemaciclib (~20%) (23,24,27). Apart from hematological toxicity, electrocardiograph (ECG) change of prolonged Q-T interval (~3%) and liver enzyme elevation (~15%, all grades) were also reported after ribociclib (24), while significantly higher incidence of diarrhoea (~10%, grade 3) was observed with abemaciclib (27). The toxicity profile of pan-PI3k inhibitor buparlisib was less favourable. There were more high-grade rash, liver enzyme derangement, hyperglycaemia, and emotional disturbance after combination buparlisib and fulvestrant (31).
Conclusions
The advent of combination hormone and novel targeted therapy has revolutionized the treatment paradigm of HR+ HER− A/MBC. CDK4/6i combined with AI as the preferred 1L hormone therapeutic option can confer ~40% ORR and median PFS of about 2 years for hormone-sensitive patients defined by ABC2 guideline. For previously-treated hormone-resistant A/MBC, ~10% ORR and >6 months median PFS can be anticipated when fulvestrant is combined with either CDK4/6i or everolimus as 2L therapy. Further improvement in outcome may need co-targeting or co-inhibition either in parallel or in series of the multiple signalling pathways which will inevitably become dynamic upon treatment exposure to hormone modulation, as well as enrichment of response by appropriate biomarker selection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO international consensus guidelines for Advanced Breast Cancer (ABC 3). Breast 2017;31:244-59. [Crossref] [PubMed]
- Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 2016;34:3069-103. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer, version 1.2017. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014;23:489-502. [Crossref] [PubMed]
- Wilcken N, Hornbuckle J, Ghersi D. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer. Cochrane Database Syst Rev 2003. [PubMed]
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233-47. [Crossref] [PubMed]
- Nabholtz JM, Buzdar A, Pollak M, et al. anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000;18:3758-67. [Crossref] [PubMed]
- Bonneterre J, Thürlimann B, Robertson JF, et al. anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 2000;18:3748-57. [Crossref] [PubMed]
- Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 2001;19:2596-606. [Crossref] [PubMed]
- Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol 2008;26:4883-90. [Crossref] [PubMed]
- Robertson JFR, Bondarenko IM, Trishkina E, et al. fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016;388:2997-3005. [Crossref] [PubMed]
- Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 2012;367:435-44. [Crossref] [PubMed]
- Bergh J, Jonsson PE, Lidbrink EK, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 2012;30:1919-25. [Crossref] [PubMed]
- Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest 2011;121:3797-803. [Crossref] [PubMed]
- Johnston SR. Enhancing endocrine therapy for hormone receptor-positive advanced breast cancer: cotargeting signaling pathways. J Natl Cancer Inst 2015;107. [Crossref] [PubMed]
- Wolff AC, Lazar AA, Bondarenko I, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 2013;31:195-202. [Crossref] [PubMed]
- Royce M, Villanueva C, Ozguroglu M, et al. BOLERO-4: Phase 2 trial of first-line everolimus plus letrozole in estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Ann Oncol 2016;27:abstr 222.
- O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 2016;13:417-30. [Crossref] [PubMed]
- Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 2016;45:129-38. [Crossref] [PubMed]
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2−negative, advanced breast cancer (PALOMA-1/ TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25-35. [Crossref] [PubMed]
- Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med 2015;373:209-19. [Crossref] [PubMed]
- Cristofanilli M, Turner NC, Bondarenko I, et al. fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425-39. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738-48. [Crossref] [PubMed]
- Tripathy D, Bardia A, Hurvitz SA, et al. Phase III randomized double-blind placebo-controlled study of ribociclib in combination with either tamoxifen and goserelin or a non-steroidal aromatase inhibitor and goserelin for the treatment of premenopausal women with HR+ HER2– advanced breast cancer: MONALEESA-7. J Clin Oncol 2017;33.
- Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875-84. [Crossref] [PubMed]
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638-46. [Crossref] [PubMed]
- Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 2012;30:2718-24. [Crossref] [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [Crossref] [PubMed]
- Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol 2014;25:2357-62. [Crossref] [PubMed]
- Baselga J, Im SA, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2−negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:904-16. [Crossref] [PubMed]
- Tobin NP, Harrell JC, Lövrot J, et al. Molecular subtype and tumor characteristics of breast cancer metastases as assessed by gene expression significantly influence patient post-relapse survival. Ann Oncol 2015;26:81-8. [Crossref] [PubMed]


