Quantitative analysis and study of the mRNA expression levels of apoptotic genes BCL2, BAX and BCL2L12 in the articular cartilage of an animal model of osteoarthritis
Introduction
Since osteoarthritis (OA) is characterized mainly by the degeneration of the articular cartilage and the loss of chondrocytes, many studies have focused on the proposition that cell death plays a central role in the course of the disease progression (1). Apoptosis and the loss of chondrocyte survival in OA results in the collapse of articular cartilage. Of course, some of the researchers point to the possibility that although apoptosis undoubtedly happens in osteoarthritic cartilage, its contribution to the breakdown of cartilage may be limited (2).
Considering the above, the research interest on the molecular signals that mediate apoptosis of articular chondrocytes is well justified. Furthermore, among the array of intracellular signals that regulate cell activity, much of the research interest is focused on the role of the BCL2 family proteins. Thus, since 1984, when the first identification of the BCL2 gene during the acute B-cell leukemia took place, a large volume of data on the function and regulation of proteins of the whole BCL2 family has been published. To this purpose, New Zealand white rabbits have been widely used as experimental animal models since they have gained favor for their numerous advantages.
Both BCL2 and BAX genes, classic members of the family, have been widely shown to act as anti- and pro-apoptotic factors, respectively (3-6). Regarding the protein BCL2L12, after its discovery in 2001, apart from the scientific team who studied it first (7), several other researchers tried to identify its role among the proteins that are involved in the control of apoptosis. So far however, the precise role of this protein in apoptotic pathways is not known yet. So, for example, several studies have shown, with clarity so far, an anti-apoptotic activity of the full-length isoform of the protein BCL2L12 in human glioblastoma cells (8-11). On the other hand, the same member appears to have a pro-apoptotic activity in human breast cancer cells (12), in fetal mouse fibroblasts (13) and in Chinese hamster ovary cells (14). The existence of such differences regarding the real role of BCL2L12 in apoptosis, as anti- or pro-apoptotic factor, it has been suggested that is cell type-dependent (13). Also, it is important that some of the isoforms of the BCL2L12 protein can be categorized in the subgroup of “BH3-only” members of the BCL2 family and hence they could act in a pro-apoptotic way, in contrast to the full-length isoform, which is most likely to inhibit apoptosis. Certainly, there is a need for further exploration of the new BCL2L12 transcripts that is necessary for our understanding about the exact role of distinct protein isoforms of BCL2L12 in apoptosis (15). In fact, a lot of protein-coding BCL2L12 transcripts have been recently cloned by members of our group, using next-generation sequencing (NGS) technology (1). NGS has significantly pioneered transcriptomics, particularly the discovery of novel transcripts (16-20).
BCL2L12 mRNA expression has been suggested as a favorable prognostic biomarker in colon cancer (21), whereas it constitutes an unfavorable and independent prognostic indicator of short-term relapse in nasopharyngeal carcinoma (22). Its potential as a molecular biomarker has also emerged in laryngeal and tongue squamous cell carcinomas (23), similar to other members of the BCL2 family which represent important molecular biomarkers in head and neck tumors (24-27), as well as in bladder cancer (28). Moreover, BCL2L12 protein overexpression predicts a favorable outcome in diffuse large B-cell lymphoma patients in the rituximab era (29), as other key members of the BCL2 apoptosis-related family (30-32). On the contrary, high BCL2L12 mRNA levels have been associated with advanced clinical stage and shown to predict shorter overall survival in chronic lymphocytic leukemia patients (33), similarly to other cancer-related genes (34-36). The prognostic significance of BCL2L12 mRNA expression in several other malignancies has been studied intensively during the last decade, similarly to other cancer-related protein-coding genes (37-42) or microRNAs (miRNAs) (43-51), along with several other clinical markers, particularly in chronic lymphocytic leukemia and myelodysplastic syndromes (52-54).
This study attempts, therefore, to improve the knowledge on the differential expression of BCL2 family genes (BCL2, BAX and BCL2L12) in the articular cartilage of an experimental animal model of OA.
Methods
Animals and tissue collection
For the purpose of this study, a total of 70 New Zealand white rabbits were used (registered supplier Trompetas A., order number EL02BIO03). All animals were kept under conventional housing conditions (single housing, 12 h light/dark, 20–21 °C temperature, 45% relative humidity, commercial rabbit food Pezzullo 12C). The operations took place under general anaesthesia of the animal (ketamine, Imalgene 70 mg/kg and xylazine, Rompun 7 mg/kg IM) and included medial patellar incision and anterior cruciate ligament transection (ACLT) to cause OA, followed by wound closure (antimicrobial: enrofloxacin—Baytril 15 mg/kg/24 h s.c./analgesia: carprofen—Rimadyl 5 mg/kg/24 h s.c.). Animals were euthanized with an intracardiac injection of pentobarbital (Dolethal) overdose under general anaesthesia. All procedures were carried out under an approved license (PD 160/1991) and in accordance with the Animals (Scientific Procedures) Act, 1986 (UK).
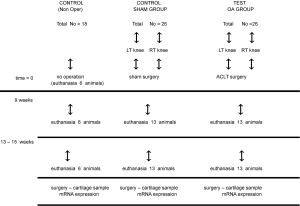
Particularly, 26 of the animals underwent an ACLT (OA group). A second group of 26 rabbits was subjected to a placebo surgery, i.e., access, cross-section of the articular membrane and wound closure including the membrane (SHAM group) and the rest of 18 specimens did not undergo an operation and constituted the control non-operated group (Figure 1).
Thirteen weeks later, all of the animals were euthanized and samples of cartilage from the osteoarthritic and non-osteoarthritic knees were collected (from both knees of each animal) (55).
Total RNA extraction and reverse transcription
Tissue specimens were homogenized and then dissolved in TRI Reagent® (Molecular Research Center, Inc., Cincinnati, OH, USA). Following the manufacturer’s instructions, total RNA was extracted from homogenized samples and was diluted in RNA Storage Solution (Life Technologies Ltd., Carlsbad, CA, USA). The concentration and purity of total RNA samples were assessed spectrophotometrically at 260 and 280 nm. Next, reverse transcription was performed using 1 µg of total RNA, M-MLV Reverse Transcriptase (Life Technologies Ltd.) as the enzyme of the reverse transcription and an oligo-dT sequence as primer. The final reaction volume was 20 µL, as previously described (56).
Quantitative real-time polymerase chain reaction (qPCR)
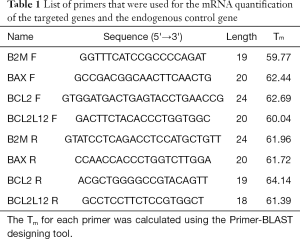
qPCR was performed using the SYBR Green chemistry in a 7500 Fast Real Time PCR System (Applied Biosystems, Foster City, CA, USA), as previously described (57,58). For this reason, a pair of gene specific primers were designed for each one of the targeted genes (Table 1) using the Pimer-BLAST algorithm. The resulting PCR amplicons for BCL2, BAX and BCL2L12 genes were 93, 174 and 107 bp long, respectively. The reaction mixture contained 1 µL of cDNA, 5 µL KAPA™ SYBR® FAST qPCR Kits (2X) (Kapa Biosystems, Inc., Woburn, MA, USA), and 2 µL of gene-specific primers (final concentration: 200 nM each), in a final reaction volume of 10 µL. The cycling conditions were as follows: a denaturation step at 95 °C for 3 min, followed by 40 cycles of 95 °C for 3 s, for denaturation of the PCR products, and 60 °C for 30 s, for primer annealing and extension (59). Each qPCR reaction was performed in duplicate to evaluate the reproducibility of data. Finally, in the current study, the human beta-2-microglobulin (B2M) gene was used as an endogenous control gene so as to normalize PCRs for the RNA amount added to the reverse transcription reactions, as previously described (60,61).
Full table
Results
The assessment of the articular cartilage condition and its alterations according to the OARSI system confirmed installed osteoarthritic lesions of varying intensity, and of grades 1 to 5, i.e., identified lesions both only in cartilage and in cartilage along with the subchondral bone.
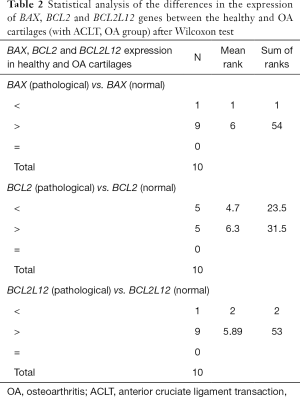
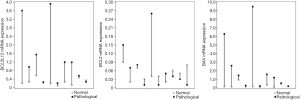
In a first analysis using the Wilcoxon test (Wilcoxon signed ranks test) on 10 pairs of rabbit cartilage samples of the OA group (animals underwent ACLT to cause OA), the difference in the expression of the three genes BAX, BCL2 and BCL2L12 between healthy and pathological cartilages was tested (Table 2) and an overexpression of BAX and BCL2L12 genes was found in ACLT-OA cartilages while the expression of BCL2 showed a variety among the different cartilages. The increased expression of BAX and BCL2L12 genes was observed in the 9 of the 10 pairs of sample in the OA group, and an increased expression of BCL2 gene was observed in only 5 of the 10 pairs of OA samples (Figure 2).
Full table
As shown in Table 3 the P values for the genes BAX (P=0.007) and BCL2L12 (P=0.009) found smaller than the minimum P value of statistical support (P=0.05), unlike for the BCL2 gene for which the P value was larger (P=0.683). Therefore, this test supports that the difference in expression of the two genes BAX and BCL2L12 is statistically significant between the healthy and the OA samples of rabbit cartilage. Contrary to these results, the difference in the expression of the BCL2 gene between the healthy and the OA samples was found statistically insignificant.
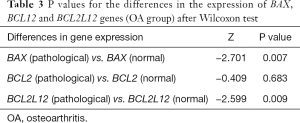
Full table
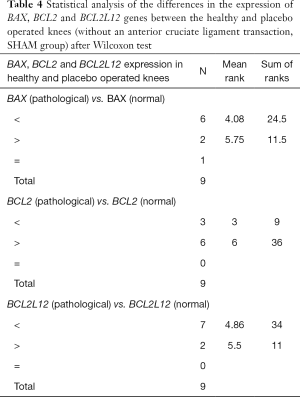
A second analysis using the Wilcoxon test on the rabbit cartilage samples of the SHAM group (placebo surgery) showed that the expression of all three genes did not change significantly between the healthy and the operated cartilages (Table 4). However, in this case, all the P values obtained for the three genes under study were larger than the statistical threshold P value. More specifically, the P values obtained were P=0.362 for BAX, P=0.108 for BCL2 and P=0.171 for BCL2L12 gene.
Full table
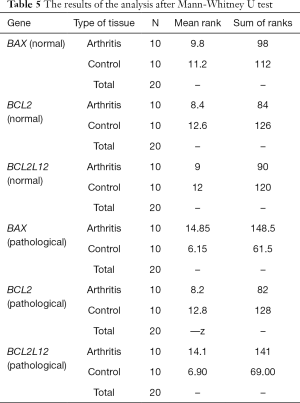
The statistical Mann-Whitney U test, in order to find possible differences in the gene expression between the healthy cartilages of both groups of samples (OA and SHAM groups) but also between the respective pathological, revealed a statistically significant difference in BAX and BCL2L12 gene expression between the operated knees with osteoarthritis and these of placebo surgery (Figure 3). More specifically, an overexpression of BAX and BCL2L12 genes was observed in the pathological cartilages with ACLT and OA in relation to pathological cartilages of the animals without ligament transection. However, this pattern was not observed in the healthy knees of the two groups, where no statistically significant differences appeared (Table 5).

Full table
Discussion
Erlacher et al. in their publication aiming to investigate the expression of BCL2 in healthy and osteoarthritic cartilages in both transcriptional and protein level, found that the BCL2 gene is expressed in both healthy and osteoarthritic cartilage and that in advanced stage OA cartilages the BCL2 gene is overexpressed (62). Feng et al., in 1998 were the first who published the direct evidence that BCL2 regulate apoptosis of articular chondrocytes (63). Two years later, Kim et al. in their publication concerning the apoptotic death of chondrocytes in OA showed not only the increased apoptosis during the progress of the disease but also the involvement of BCL2, BAX and Fas. Indeed, similar to Feng et al., this research showed lower levels of BCL2 in OA cartilages compared to the healthy ones but significantly higher expression of BCL2 in OA cartilages of advanced stage than in OA cartilages without serious lesions and constant levels of BAX (64). In 2004, Mistry et al. showed that despite the fact that both BCL2 and BAX were detected easily in the chondrocytes of tibial cartilages of mice, there were no changes indicating the activation of an intracellular apoptotic pathway. However, according to the same researchers, it is important to emphasize that, at best, immunohistochemistry is only a semiquantitative technique and in the absence of marked changes in BCL2 relative to BAX, no conclusion as to activation of this pathway is justified (65). Iannone et al., in 2005 published a study aiming to identify the role of p53, BCL2 and Fas/CD95 in controlling the metabolism of cartilage. According to their findings, BCL2 and p53 play a role in apoptosis, but also can help in regulating the growth and differentiation of chondrocytes. The critical conclusion from this study was that the ratio of BCL2/p53 was increased in OA cartilage due to the increase of BCL2 and the decrease of p53 levels in chondrocytes (66). In 2009, Lin et al. studied the effects of kneepad on expression of BCL2 and TP53 mRNA of chondrocyte in white rabbits with knee OA, so as to explore and treatment mechanism of OA kneepad on apoptosis of chondrocytes of rabbits with knee OA in molecular degree. Their results showed that OA-kneepad can up-regulate the mRNA expression of BCL2 as well as down-regulate the mRNA expression of TP53, thereby to inhibit the apoptosis of cartilage cells and delay the degeneration of articular cartilage changes (67).
In 2015, in the publication of Karaliotas et al., the expression analysis of BCL2, BAX and BCL2L12 apoptotic genes, showed that these genes are indeed expressed in cartilaginous tissue and secondly that there are differences in the expression of these three between osteoarthritic and non-osteoarthritic cartilage. More specifically, in comparison with the non-osteoarthritic cartilages, in the osteoarthritic samples the mRNA levels of both the pro-apoptotic gene BAX and the gene BCL2L12 were increased, while the mRNA levels of the anti-apoptotic gene BCL2 were downregulated. The results showed that the mRNA expression of BAX gene appear to have an increasing trend in the OA cartilages compared with the healthy ones, although without statistical significance. In contrast, the ratio of BCL2/BAX gene expression was found to be significantly decreased in the OA cartilages compared with the healthy ones. Furthermore, patients with OA of stage III showed an important overexpression of BAX in comparison with the ones of the control group, while the ratio of BCL2/BAX gene expression was markedly decreased. On the other hand, both control and OA groups showed a positive correlation between the mRNA levels of BAX and BCL2. According to the researchers, these results further implicate apoptosis in the pathogenesis of OA, through molecular mechanisms, which include the aberrant expression of the BCL2 gene family (68), including BCL2L12.
BCL2L12 is located on the chromosomal region 19q13.3-q13.4, at a distance of 7.5 Mb from the telomeres of the long arm of chromosome 19, close to many cancer-related genes such as the cluster of tissue kallikrein and kallikrein-related peptidases (69,70), a family of peptidases with important properties as biomarkers (71). To date, several miRNAs have been shown to regulate expression of BCL2L12 and/or other members of the BCL2 family; most of them constitute important biomarkers (48,72-81).
In the present study, according to the statistical analysis of the expression levels of BAX, BCL2 and BCL2L12 in healthy and OA cartilages using the Wilcoxon test, BAX and BCL2L12 genes showed an overexpression in OA cartilages, while no conclusion could be exported from the statistically insignificant differences of BCL2 expression. This result indicates the change of the expression of the BAX and BCL2L12 genes, after the induction of OA and therefore in the apoptosis of the articular chondrocytes. However, it is necessary to underline that the main and significant factor for the control of the viability of human articular chondrocytes are not the absolute values of the expression of each protein but the proportions of the expression of anti- and pro-apoptotic members, following the results of previous researchers such as Feng et al. (63).
These differences in the expression of BAX, BCL12 and BCL2L12 genes between the healthy and OA cartilages clearly demonstrate that the expression of BAX and BCL2L12 is upregulated by signals that induce apoptosis in the articular chondrocytes while changes in the expression of BCL2 are not supported by statistics. Although Feng et al. observed the opposite expression settings, i.e., upregulation of the BCL2 expression and stable BAX expression, in both studies, the ratio BAX/BCL2 expression is increased in OA and therefore promotes the chondrocyte apoptosis. Of course, the findings of Feng et al. confirm a direct implication of BCL2 in the regulation of apoptosis of articular chondrocytes. According to the findings of the present study this implication appeared indirect, but still the differences in the expression of BCL2 between the healthy and OA samples are not supported statistically. Here, a more direct role of BAX and BCL2L12 in the regulation of apoptosis of articular chondrocytes seems to take the place of BCL2.
During the progression of OA, the promotion of the apoptotic process and the predominance of the pro-apoptotic versus the anti-apoptotic BCL2 family members are naturally expected. Trying to interpret the increase in the expression of the classic pro-apoptotic protein BAX no particular problems exist, since all bibliographic data agree that the development of OA is accompanied by the promotion of the apoptotic process and most of them confirm the overexpression of BAX in tissues subjected to the apoptotic death. On the other hand, the absence of statistical support in the differences of expression of the anti-apoptotic member BCL2 does not permit the creation of any hypothesis and of course the export of safe conclusions.
Regarding the overexpression of BCL2L12 in osteoarthritic cartilages, the existence of published results from other tissues, despite their variety and differentiation depending on tissue type, gives the chance to interpret the results of the present study under the premise of two separate scenarios: The first is that the role of BCL2L12, pro- or anti-apoptotic, may present specialization per cell type. Thus, differences in the expression and action of this BCL2 family member, in the way it is presented by different publications, is finally cell type-dependent (13). This argues that in the osteoarthritis cartilage, BCL2L12 role, as shown by its expression levels, is pro-apoptotic. However, the very recent proof of the existence of a lot of different BCL2L12 protein isoforms (1), which because of their different structure (for example isoforms BCL2L12 are characterized as BH3-only) are expected to have different potency, it is not only the second premise for the interpretation of the BCL2L12 overexpression in osteoarthritic cartilage but it could partly interpret the first assumption of cell dependent action. The increased expression levels of BCL2L12 in the osteoarthritic cartilage, correspond rather to one of the protein isoforms with a pro-apoptotic role.
Unlike the comparison between the healthy and osteoarthritis cartilages, according to the statistical analysis of the difference in the expression of BAX, BCL12 and BCL2L12 genes between healthy and pathological (placebo operated and not osteoarthritic) cartilages, showed that the expression of all three genes did not significantly vary. This result gives value to the findings of the previous comparison and partly supports any hypothesis about the differential gene expression between osteoarthritic and healthy samples. Furthermore, the statistically significant difference in BAX and BCL2L12 gene expression between the OA and SHAM cartilages, while this is not observed in healthy samples, also supports the validity of the first analysis.
According to Iannone et al. (66), the differentiation of the results among the different publications on the role of BCL2 family members in chondrocyte apoptosis, can be attributed to the different age of patients and donors, because the expression of these proteins by chondrocytes depends on age (82), the degree and the progression of the disease and the different quantification methodologies. So, the study of the expression of anti-and pro-apoptotic proteins into active chondrocytes of an osteoarthritic cartilage will lead to conflicting results, depending on the relative prevalence of proliferation or apoptosis in this particular stage of the disease and in such specified area of cartilage (66).
Since apoptosis is the main feature of the degeneration of the cartilage in OA, the effective inhibition of apoptosis of chondrocytes could provide new and interesting ideas in a therapeutic strategy for the treatment of the disease. The results of this study indicate the pro-apoptotic action of BAX and BCL2L12 but not the generally acceptable anti-apoptotic effect of BCL2. Although the therapeutic targets in several types of cancer are the anti-apoptotic activities that maintain the survival of cancer cells, in the case of OA, the survival of the chondrocytes is crucial for the inhibition of progression of the disease but also for healing. In OA, the treatment goals are changed completely and now are not the anti- but the pro-apoptotic actions that promote cell death. So the results of this study highlight the BCL2 family members BAX and BCL2L12 as potential therapeutic targets in OA. However, a restriction is the fact of non-identification of the particular protein isoform or isoforms of BCL2L12 which in this case showed a pro-apoptotic action. On the other hand, this restriction is also a candidate question for a future research.
Conclusions
While the chondrocyte apoptosis is one of the most important factors in the pathogenesis of OA the focus of the research interest on molecular signals that mediate apoptosis of the articular chondrocytes represents progress in the efforts to find new therapeutic targets. The assessment of articular cartilage condition and lesions with the OARSI system confirmed installed osteoarthritic lesions of varying intensity, grades 1 to 5, indicating that the method of ACLT is an effective mean of causing OA in white New Zealand rabbits.
In addition, the differences between the expression of BAX, BCL12 and BCL2L12 between the healthy and OA cartilages show that the expression of BAX and BCL2L12 is upregulated by signals that induce apoptosis in chondrocytes while changes in the expression of BCL2 are not statistically supported. During the progression of OA, the promotion of the apoptotic process and the predominance of pro- versus anti-apoptotic BCL2 family members are naturally expected. In trying to interpret the increase in the expression of pro-apoptotic protein BAX no particular problems are present. Furthermore, the absence of statistical support in the differences of expression of the anti-apoptotic member BCL2 does not permit the creation of any hypothesis and of course the export of safe conclusions. The very recent proof of the existence of many different protein isoforms of BCL2L12 which, because of their different structure are expected to have different action, could interpret partly the assumption of cell type-dependent action of this member. Certainly, there is a need for further investigation for new transcripts of BCL2L12 that is necessary for our understanding about the exact role of distinct protein isoforms of BCL2L12 in apoptosis and the induction or progression of OA. Since apoptosis is the main feature of the degeneration of the cartilage in OA, the effective inhibition of apoptosis of chondrocytes can provide new and interesting ideas in a therapeutic strategy for the treatment of OA. In the case of OA, the survival of the cells is crucial for inhibition of progression of the disease but also for healing. The therapeutic targets need to be the pro-apoptotic actions that promote cell death. Thus, BAX and BCL2L12 are highlighted as potential therapeutic targets in OA.
Acknowledgements
This study was supported by the National and Kapodistrian University of Athens, Special Account of Research Grants, Experimental & Research Center of ELPEN S.A., Athens Club and by the A. G. Leventis Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures were carried out under an approved license (PD 160/1991) and in accordance with the Animals (Scientific Procedures) Act, 1986 (UK).
References
- Adamopoulos PG, Kontos CK, Tsiakanikas P, et al. Identification of novel alternative splice variants of the BCL2L12 gene in human cancer cells using next-generation sequencing methodology. Cancer Lett 2016;373:119-29. [Crossref] [PubMed]
- Aigner T, Kim HA. Apoptosis and cellular vitality: issues in osteoarthritic cartilage degeneration. Arthritis Rheum 2002;46:1986-96. [Crossref] [PubMed]
- Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci 2006;43:1-67. [Crossref] [PubMed]
- Green DR, Chipuk JE. Apoptosis: Stabbed in the BAX. Nature 2008;455:1047-9. [Crossref] [PubMed]
- Fletcher JI, Meusburger S, Hawkins CJ, et al. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci U S A 2008;105:18081-7. [Crossref] [PubMed]
- Stamati L, Avgeris M, Kosmidis H, et al. Overexpression of BCL2 and BAX following BFM induction therapy predicts ch-ALL patients' poor response to treatment and short-term relapse. J Cancer Res Clin Oncol 2015;141:2023-36. [Crossref] [PubMed]
- Scorilas A, Kyriakopoulou L, Yousef GM, et al. Molecular cloning, physical mapping, and expression analysis of a novel gene, BCL2L12, encoding a proline-rich protein with a highly conserved BH2 domain of the Bcl-2 family. Genomics 2001;72:217-21. [Crossref] [PubMed]
- Stegh AH, Kesari S, Mahoney JE, et al. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci U S A 2008;105:10703-8. [Crossref] [PubMed]
- Stegh AH, Kim H, Bachoo RM, et al. Bcl2L12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes Dev 2007;21:98-111. [Crossref] [PubMed]
- Chou CH, Chou AK, Lin CC, et al. GSK3beta regulates Bcl2L12 and Bcl2L12A anti-apoptosis signaling in glioblastoma and is inhibited by LiCl. Cell Cycle 2012;11:532-42. [Crossref] [PubMed]
- Yang MC, Loh JK, Li YY, et al. Bcl2L12 with a BH3-like domain in regulating apoptosis and TMZ-induced autophagy: a prospective combination of ABT-737 and TMZ for treating glioma. Int J Oncol 2015;46:1304-16. [Crossref] [PubMed]
- Hong Y, Yang J, Wu W, et al. Knockdown of BCL2L12 leads to cisplatin resistance in MDA-MB-231 breast cancer cells. Biochim Biophys Acta 2008;1782:649-57. [Crossref] [PubMed]
- Nakajima A, Nishimura K, Nakaima Y, et al. Cell type-dependent proapoptotic role of Bcl2L12 revealed by a mutation concomitant with the disruption of the juxtaposed Irf3 gene. Proc Natl Acad Sci U S A 2009;106:12448-52. [Crossref] [PubMed]
- Hong Y, Yang J, Chi Y, et al. BCL2L12A localizes to the cell nucleus and induces growth inhibition through G2/M arrest in CHO cells. Mol Cell Biochem 2010;333:323-30. [Crossref] [PubMed]
- Kontos CK, Scorilas A. Molecular cloning of novel alternatively spliced variants of BCL2L12, a new member of the BCL2 gene family, and their expression analysis in cancer cells. Gene 2012;505:153-66. [Crossref] [PubMed]
- Adamopoulos PG, Kontos CK, Scorilas A. Novel splice variants of the human kallikrein-related peptidases 11 (KLK11) and 12 (KLK12), unraveled by Next-Generation Sequencing technology. Biol Chem 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Adamopoulos PG, Kontos CK, Scorilas A. Identification and molecular cloning of novel transcripts of the human kallikrein-related peptidase 10 (KLK10) gene using next-generation sequencing. Biochem Biophys Res Commun 2017;487:776-81. [Crossref] [PubMed]
- Adamopoulos PG, Kontos CK, Scorilas A. Molecular cloning of novel transcripts of human kallikrein-related peptidases 5, 6, 7, 8 and 9 (KLK5 - KLK9), using Next-generation sequencing. Sci Rep 2017;7:17299. [Crossref] [PubMed]
- Adamopoulos PG, Kontos CK, Scorilas A. Discovery of novel transcripts of the human tissue kallikrein (KLK1) and kallikrein-related peptidase 2 (KLK2) in human cancer cells, exploiting Next-Generation Sequencing technology. Genomics 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Parmakelis A, Kotsakiozi P, Kontos CK, et al. The transcriptome of a "sleeping" invader: de novo assembly and annotation of the transcriptome of aestivating Cornu aspersum. BMC Genomics 2017;18:491. [Crossref] [PubMed]
- Kontos CK, Papadopoulos IN, Scorilas A. Quantitative expression analysis and prognostic significance of the novel apoptosis-related gene BCL2L12 in colon cancer. Biol Chem 2008;389:1467-75. [Crossref] [PubMed]
- Fendri A, Kontos CK, Khabir A, et al. BCL2L12 is a novel biomarker for the prediction of short-term relapse in nasopharyngeal carcinoma. Mol Med 2011;17:163-71. [Crossref] [PubMed]
- Geomela PA, Kontos CK, Yiotakis I, et al. Quantitative expression analysis of the apoptosis-related gene, BCL2L12, in head and neck squamous cell carcinoma. J Oral Pathol Med 2013;42:154-61. [Crossref] [PubMed]
- Fendri A, Kontos CK, Khabir A, et al. Quantitative analysis of BCL2 mRNA expression in nasopharyngeal carcinoma: an unfavorable and independent prognostic factor. Tumour Biol 2010;31:391-9. [Crossref] [PubMed]
- Giotakis AI, Kontos CK, Manolopoulos LD, et al. High BAX/BCL2 mRNA ratio predicts favorable prognosis in laryngeal squamous cell carcinoma, particularly in patients with negative lymph nodes at the time of diagnosis. Clin Biochem 2016;49:890-6. [Crossref] [PubMed]
- Kontos CK, Fendri A, Khabir A, et al. Quantitative expression analysis and prognostic significance of the BCL2-associated X gene in nasopharyngeal carcinoma: a retrospective cohort study. BMC Cancer 2013;13:293. [Crossref] [PubMed]
- Kontos CK, Adamopoulos PG, Scorilas A. Molecular biomarkers of laryngeal cancer. In: Biomarkers in Disease: Methods, Discoveries and Applications: Biomarkers in Cancer. Netherlands: Springer, 2015:891-919.
- Foutadakis S, Avgeris M, Tokas T, et al. Increased BCL2L12 expression predicts the short-term relapse of patients with TaT1 bladder cancer following transurethral resection of bladder tumors. Urol Oncol 2014;32:39.e29-36. [Crossref] [PubMed]
- Papageorgiou SG, Kontos CK, Foukas PG, et al. BCL2L12 protein overexpression is associated with favorable outcome in diffuse large B-cell lymphoma patients in the rituximab era. Leuk Lymphoma 2016;57:2199-203. [Crossref] [PubMed]
- Christodoulou MI, Kontos CK, Halabalaki M, et al. Nature promises new anticancer agents: Interplay with the apoptosis-related BCL2 gene family. Anticancer Agents Med Chem 2014;14:375-99. [Crossref] [PubMed]
- Kontos CK, Christodoulou MI, Scorilas A. Apoptosis-related BCL2-family members: Key players in chemotherapy. Anticancer Agents Med Chem 2014;14:353-74. [Crossref] [PubMed]
- Tarushi A, Raptopoulou CP, Psycharis V, et al. Copper(II) Inverse-[9-Metallacrown-3] Compounds Accommodating Nitrato or Diclofenac Ligands: Structure, Magnetism, and Biological Activity. Eur J Inorg Chem 2016;2016:219-31. [Crossref]
- Papageorgiou SG, Kontos CK, Pappa V, et al. The novel member of the BCL2 gene family, BCL2L12, is substantially elevated in chronic lymphocytic leukemia patients, supporting its value as a significant biomarker. Oncologist 2011;16:1280-91. [Crossref] [PubMed]
- Adamopoulos PG, Kontos CK, Papageorgiou SG, et al. KLKB1 mRNA overexpression: A novel molecular biomarker for the diagnosis of chronic lymphocytic leukemia. Clin Biochem 2015;48:849-54. [Crossref] [PubMed]
- Kontos CK, Adamopoulos PG, Papageorgiou SG, et al. mRNA overexpression of kallikrein-related peptidase 14 (KLK14) is an independent predictor of poor overall survival in chronic lymphocytic leukemia patients. Clin Chem Lab Med 2016;54:315-24. [Crossref] [PubMed]
- Kontos CK, Papageorgiou SG, Diamantopoulos MA, et al. mRNA overexpression of the hypoxia inducible factor 1 alpha subunit gene (HIF1A): An independent predictor of poor overall survival in chronic lymphocytic leukemia. Leuk Res 2017;53:65-73. [Crossref] [PubMed]
- Kefala M, Papageorgiou SG, Kontos CK, et al. Increased expression of phosphorylated NBS1, a key molecule of the DNA damage response machinery, is an adverse prognostic factor in patients with de novo myelodysplastic syndromes. Leuk Res 2013;37:1576-82. [Crossref] [PubMed]
- Kontos CK. Surrogate Prognostic Biomarkers in OSCC: The Paradigm of PA28gamma Overexpression. EBioMedicine 2015;2:784-5. [Crossref] [PubMed]
- Kontos CK, Scorilas A, Papavassiliou AG. The role of transcription factors in laboratory medicine. Clin Chem Lab Med 2013;51:1563-71. [PubMed]
- Psyrri A, Papageorgiou S, Liakata E, et al. Phosphatidylinositol 3'-kinase catalytic subunit alpha gene amplification contributes to the pathogenesis of mantle cell lymphoma. Clin Cancer Res 2009;15:5724-32. [Crossref] [PubMed]
- Vassilakopoulou M, Avgeris M, Velcheti V, et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin Cancer Res 2016;22:704-13. [Crossref] [PubMed]
- Miltiades P, Lamprianidou E, Vassilakopoulos TP, et al. The Stat3/5 Signaling Biosignature in Hematopoietic Stem/Progenitor Cells Predicts Response and Outcome in Myelodysplastic Syndrome Patients Treated with Azacitidine. Clin Cancer Res 2016;22:1958-68. [Crossref] [PubMed]
- Kontos CK, Vasilatou D, Papageorgiou SG, et al. Translation Regulation by microRNAs in Acute Leukemia. Reviews in Cell Biology and Molecular Medicine. Wiley-VCH Verlag GmbH & Co. KGaA, 2014.
- Skourti E, Logotheti S, Kontos CK, et al. Progression of mouse skin carcinogenesis is associated with the orchestrated deregulation of mir-200 family members, mir-205 and their common targets. Mol Carcinog 2016;55:1229-42. [Crossref] [PubMed]
- Vasilatou D, Papageorgiou SG, Kontsioti F, et al. Expression analysis of mir-17-5p, mir-20a and let-7a microRNAs and their target proteins in CD34+ bone marrow cells of patients with myelodysplastic syndromes. Leuk Res 2013;37:251-8. [Crossref] [PubMed]
- Tsikrika FD, Avgeris M, Levis PK, et al. miR-221/222 cluster expression improves clinical stratification of non-muscle invasive bladder cancer (TaT1) patients' risk for short-term relapse and progression. Genes Chromosomes Cancer 2018;57:150-61. [Crossref] [PubMed]
- Piatopoulou D, Avgeris M, Marmarinos A, et al. miR-125b predicts childhood acute lymphoblastic leukaemia poor response to BFM chemotherapy treatment. Br J Cancer 2017;117:801-12. [Crossref] [PubMed]
- Kontos CK, Tsiakanikas P, Avgeris M, et al. miR-15a-5p, A Novel Prognostic Biomarker, Predicting Recurrent Colorectal Adenocarcinoma. Mol Diagn Ther 2017;21:453-64. [Crossref] [PubMed]
- Avgeris M, Mavridis K, Tokas T, et al. Uncovering the clinical utility of miR-143, miR-145 and miR-224 for predicting the survival of bladder cancer patients following treatment. Carcinogenesis 2015;36:528-37. [Crossref] [PubMed]
- Avgeris M, Stravodimos K, Scorilas A. Loss of miR-378 in prostate cancer, a common regulator of KLK2 and KLK4, correlates with aggressive disease phenotype and predicts the short-term relapse of the patients. Biol Chem 2014;395:1095-104. [Crossref] [PubMed]
- Avgeris M, Stravodimos K, Fragoulis EG, et al. The loss of the tumour-suppressor miR-145 results in the shorter disease-free survival of prostate cancer patients. Br J Cancer 2013;108:2573-81. [Crossref] [PubMed]
- Mpakou VE, Ioannidou HD, Konsta E, et al. Quantitative and qualitative analysis of regulatory T cells in B cell chronic lymphocytic leukemia. Leuk Res 2017;60:74-81. [Crossref] [PubMed]
- Papageorgiou SG, Vasilatou D, Kontos CK, et al. Treatment with 5-Azacytidine improves clinical outcome in high-risk MDS patients in the 'real life' setting: A single center observational study. Hematology 2016;21:34-41. [Crossref] [PubMed]
- Papageorgiou SG, Vasilatou D, Kontos CK, et al. The prognostic value of monosomal karyotype (MK) in higher-risk patients with myelodysplastic syndromes treated with 5-Azacitidine: A retrospective analysis of the Hellenic (Greek) Myelodysplastic syndromes Study Group. Am J Hematol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Tiraloche G, Girard C, Chouinard L, et al. Effect of oral glucosamine on cartilage degradation in a rabbit model of osteoarthritis. Arthritis Rheum 2005;52:1118-28. [Crossref] [PubMed]
- Alexopoulou DK, Kontos CK, Christodoulou S, et al. KLK11 mRNA expression predicts poor disease-free and overall survival in colorectal adenocarcinoma patients. Biomark Med 2014;8:671-85. [Crossref] [PubMed]
- Kontos CK, Chantzis D, Papadopoulos IN, et al. Kallikrein-related peptidase 4 (KLK4) mRNA predicts short-term relapse in colorectal adenocarcinoma patients. Cancer Lett 2013;330:106-12. [Crossref] [PubMed]
- Foteinou E, Kontos CK, Giotakis AI, et al. Low mRNA expression levels of kallikrein-related peptidase 4 (KLK4) predict short-term relapse in patients with laryngeal squamous cell carcinoma. Biol Chem 2014;395:1051-62. [Crossref] [PubMed]
- Christodoulou S, Alexopoulou DK, Kontos CK, et al. Kallikrein-related peptidase-6 (KLK6) mRNA expression is an independent prognostic tissue biomarker of poor disease-free and overall survival in colorectal adenocarcinoma. Tumour Biol 2014;35:4673-85. [Crossref] [PubMed]
- Geomela PA, Kontos CK, Yiotakis I, et al. L-DOPA decarboxylase mRNA expression is associated with tumor stage and size in head and neck squamous cell carcinoma: a retrospective cohort study. BMC Cancer 2012;12:484. [Crossref] [PubMed]
- Kontos CK, Papadopoulos IN, Fragoulis EG, et al. Quantitative expression analysis and prognostic significance of L-DOPA decarboxylase in colorectal adenocarcinoma. Br J Cancer 2010;102:1384-90. [Crossref] [PubMed]
- Erlacher L, Maier R, Ullrich R, et al. Differential expression of the protooncogene bcl-2 in normal and osteoarthritic human articular cartilage. J Rheumatol 1995;22:926-31. [PubMed]
- Feng L, Precht P, Balakir R, et al. Evidence of a direct role for Bcl-2 in the regulation of articular chondrocyte apoptosis under the conditions of serum withdrawal and retinoic acid treatment. J Cell Biochem 1998;71:302-9. [Crossref] [PubMed]
- Kim HA, Lee YJ, Seong SC, et al. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol 2000;27:455-62. [PubMed]
- Mistry D, Oue Y, Chambers MG, et al. Chondrocyte death during murine osteoarthritis. Osteoarthritis Cartilage 2004;12:131-41. [Crossref] [PubMed]
- Iannone F, De Bari C, Scioscia C, et al. Increased Bcl-2/p53 ratio in human osteoarthritic cartilage: a possible role in regulation of chondrocyte metabolism. Ann Rheum Dis 2005;64:217-21. [Crossref] [PubMed]
- Lin MN, Liu XX, Wang SL, et al. Effect of OA kneepad on apoptosis genes Bcl-2 and p53 expression in articular cartilage cells of experimental knee osteoarthritis. Zhongguo Gu Shang 2009;22:688-91. [PubMed]
- Karaliotas GI, Mavridis K, Scorilas A, et al. Quantitative analysis of the mRNA expression levels of BCL2 and BAX genes in human osteoarthritis and normal articular cartilage: An investigation into their differential expression. Mol Med Rep 2015;12:4514-21. [Crossref] [PubMed]
- Kontos CK, Mavridis K, Talieri M, et al. Kallikrein-related peptidases (KLKs) in gastrointestinal cancer: mechanistic and clinical aspects. Thromb Haemost 2013;110:450-7. [Crossref] [PubMed]
- Kontos CK, Scorilas A. Kallikrein-related peptidases (KLKs): a gene family of novel cancer biomarkers. Clin Chem Lab Med 2012;50:1877-91. [Crossref] [PubMed]
- Kontos CK, Adamopoulos PG, Scorilas A. Prognostic and predictive biomarkers in prostate cancer. Expert Rev Mol Diagn 2015;15:1567-76. [Crossref] [PubMed]
- Kerimis D, Kontos CK, Christodoulou S, et al. Elevated expression of miR-24-3p is a potentially adverse prognostic factor in colorectal adenocarcinoma. Clin Biochem 2017;50:285-92. [Crossref] [PubMed]
- Papageorgiou SG, Kontos CK, Tsiakanikas P, et al. Elevated miR-20b-5p expression in peripheral blood mononuclear cells: A novel, independent molecular biomarker of favorable prognosis in chronic lymphocytic leukemia. Leuk Res 2018;70:1-7. [Crossref] [PubMed]
- Rapti SM, Kontos CK, Papadopoulos IN, et al. Enhanced miR-182 transcription is a predictor of poor overall survival in colorectal adenocarcinoma patients. Clin Chem Lab Med 2014;52:1217-27. [Crossref] [PubMed]
- Tsiakanikas P, Kontos CK, Kerimis D, et al. High microRNA-28-5p expression in colorectal adenocarcinoma predicts short-term relapse of node-negative patients and poor overall survival of patients with non-metastatic disease. Clin Chem Lab Med 2018;56:990-1000. [Crossref] [PubMed]
- Rapti SM, Kontos CK, Papadopoulos IN, et al. High miR-96 levels in colorectal adenocarcinoma predict poor prognosis, particularly in patients without distant metastasis at the time of initial diagnosis. Tumour Biol 2016;37:11815-24. [Crossref] [PubMed]
- Papageorgiou SG, Kontos CK, Diamantopoulos MA, et al. MicroRNA-155-5p Overexpression in Peripheral Blood Mononuclear Cells of Chronic Lymphocytic Leukemia Patients Is a Novel, Independent Molecular Biomarker of Poor Prognosis. Dis Markers 2017;2017. [Crossref] [PubMed]
- Rapti SM, Kontos CK, Christodoulou S, et al. miR-34a overexpression predicts poor prognostic outcome in colorectal adenocarcinoma, independently of clinicopathological factors with established prognostic value. Clin Biochem 2017;50:918-24. [Crossref] [PubMed]
- Adamopoulos PG, Kontos CK, Rapti SM, et al. miR-224 overexpression is a strong and independent prognosticator of short-term relapse and poor overall survival in colorectal adenocarcinoma. Int J Oncol 2015;46:849-59. [Crossref] [PubMed]
- Diamantopoulos MA, Kontos CK, Kerimis D, et al. Upregulated miR-16 expression is an independent indicator of relapse and poor overall survival of colorectal adenocarcinoma patients. Clin Chem Lab Med 2017;55:737-47. [Crossref] [PubMed]
- Ferraro A, Kontos CK, Boni T, et al. Epigenetic regulation of miR-21 in colorectal cancer: ITGB4 as a novel miR-21 target and a three-gene network (miR-21-ITGBeta4-PDCD4) as predictor of metastatic tumor potential. Epigenetics 2014;9:129-41. [Crossref] [PubMed]
- Aigner T, Hemmel M, Neureiter D, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum 2001;44:1304-12. [Crossref] [PubMed]