miR-486 inhibits PM2.5-induced apoptosis and oxidative stress in human lung alveolar epithelial A549 cells
Introduction
Known as the particles and droplets with aerodynamic diameter ≤2.5 µm, PM2.5 accounts for a significant proportion of pollution in developing countries and regions (1). The association between disease occurrence and PM2.5 exposure have confirmed in many studies, especially respiratory diseases (2-4). Although significant efforts have been made to understand the lung damages induced by PM2.5, the underlying biological processes and mechanisms are rarely known. Accumulating studies have shown that cell apoptosis, cell necrosis, autophagy, DNA damages, mitochondria damage and gene mutations contribute to PM2.5-induced cytotoxicity (5-7). Several signaling pathways have been reported to be involved in these biological processes. Nevertheless, the epigenetic regulation, especially the roles of miRNAs in PM2.5-induced cell injury are still to be elucidated.
MicroRNAs (miRNAs, miRs) are ~21–23-nucleotide single-stranded RNAs that play tremendous regulatory roles in many biological processes. MiRNAs can regulate gene expression both in transcriptional level and posttranscriptional level. Increasing evidence suggests that miRNAs contribute to a spectrum of cell fate, including cell survival and cell death. Several studies have shown that the expression of miRNAs is altered after PM2.5 exposure, but the underlying mechanisms are still unclear (8,9). MiR-486 has been reported to inhibit mitochondrial mediated apoptotic pathway and protect against H2O2-stimulated cardiomyocyte deaths (10). Also, miR-486-5p-containing exosomes treatment is demonstrated to be an effective strategy to protect against endothelial injury (11). However, the roles of miR-486 in PM2.5-induced lung alveolar epithelial cell injury are still unknown.
In this study, miR-486 was found decreased in human lung alveolar epithelial A549 cells after incubated with PM2.5. Further data showed that miR-486 mimic administration inhibited PM2.5-induced cell injury in A549 cells. Moreover, we demonstrated that miR-486 exerted cell-protecting effects through targeting PTEN and FOXO1. Overall, our study suggests that miR-486 prevents human lung alveolar epithelial cells from PM2.5-induced cell damages.
Methods
Materials and regents
PM2.5 (NIST, USA), Lipofectamine™ 2000 Transfection Reagent (Invitrogen, USA), TUNEL FITC Apoptosis Detection Kit (Vazyme, China), and ROS Assay Kit (Beyotime, China) were used.
PTEN overexpression plasmid-The CDS sequences was determined in NCBI. And PTEN CDS sequence was ligated into FUGW after amplified by PCR. PCR primers we used were as follows (the italic part was the digestion sequences of restriction enzyme): Forward primer: 5'-GGGGGATCCATGACAGCCATCATCAA
AGAGATCG-3'(BamH1); Reverse primer: 5'-GGGCTCGAGTCAGACTTTTGTA
ATTTGTGTATG-3'(Xho1). And FUGW used as a control plasmid.
The miR-486mimic, miR-486 inhibitor and siRNA FOXO1 purchase from RiboBio Co., Ltd (RiboBio, China).
PM2.5 preparation
The 50 mg PM2.5 particle was dissolved in 1 mL DMSO, then the mixture treated with ultrasonic vibration in Ultrasonic oscillometer for 20 min. Then the mixture was purified in centrifugal machine at 17,000 g and 4 °C for 10 min.
Cell culture and transfection
A549 cells were cultured in the environment at 37 °C with 5% CO2. And nutrient media components were Dulbecco modified Eagle medium (DMEM), 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS).
The transfection of A549 cells was carried out 24 h after cell seeding. Lipofectamine™ 2000 was used as transfection reagents following manufacturer’s instructions. Firstly, PTEN overexpressing plasmid, FOXO siRNA, miR-486 mimic or inhibitor was mix with Lipofectamine™ 2000 transfection reagent. Then the mixture was added to medium. Fresh medium was changed after 6–10 hours.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Isolation and purification the total RNA from cells using RNAiso Plus (Takara, Japan) and then reverse-transcribed into cDNA by RevertAid First Strand cDNA Synthesis Kit (Invitrogen, USA). miRNA qPCR Primer (RiboBio, China) was used to determine the expression of miR-486 by Takara SYBR Premix Ex Taq (Takara, China) in a Roche Real-Time PCR Detection System. 5S was used as an internal control.
TUNEL staining
TUNEL staining was performed according to the instruction manual. In brief, 4% PFA was used to fix the A549 cells and then stained with TUNEL staining buffer. Cell nucleus was stained with Hoechst dissolved in 5% BSA for 20 min at room temperature in darkness. Digital images were acquired by fluorescence microscope (Leica, Germany). TUNEL positive cells were counted with Image J software.
Western blot
Protein was extracted from cells after treatment. SDS-PAGE was used to separate the proteins and then the PVDF membrane (Millipore, USA) was used to blot the protein from polyacrylamide gel. The membrane was incubated with primary antibody at 4 °C overnight after blocked with 5% BSA. The appropriate HRP-conjugated secondary antibodies were used to amplify the signals of primary antibodies. Then the ECL western blotting detection reagent (Tanon, China) was used to display the content of protein and quantified by Image J Software. The antibodies of GAPDH (ABclonal, USA), Bax (ABclonal, USA) and Bcl-2 (ABclonal, USA) were used in this work.
ROS detection
The ROS generation of cells was measured by ROS Assay Kit (Beyotime, China). Specifically, after treatment, A549 cells were washed with PBS and then incubated with 10 µM DCFH-DA and 2 µg/mL Hoechst 33342 for 20 min. Digital images were acquired with fluorescence microscope. The fluorescence intensity was analyzed with Image J Software.
Statistical analysis
All data was expressed as mean ± SEM. All statistical analyses were performed through IBM SPSS Statistics 20.0. Variables between groups were subject to independent sample t-test. One-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test was used for multiple comparisons. P value less than 0.05 was considered statistically significant.
Results
miR-486 reduces PM2.5-induced cell apoptosis in human lung alveolar epithelial A549 cells
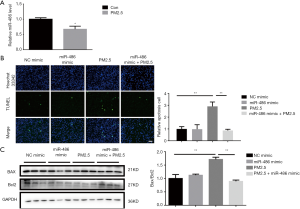
Human lung alveolar epithelial A549 cells were incubated with PM2.5 at concentration of 50 µg/mL. We firstly compared the expression of miR-486 in PM2.5-treated A549 cells and normal A549 cells. The expression of miR-486 was dramatically decreased after PM2.5 incubated (Figure 1A), which suggested that the miR-486 may protect against PM2.5-induced cell injury. In order to confirm the potential protective effects of miR-486 in a PM2.5 environment, miR-486 mimic was used to up-regulate the expression of miR-486 in A549 cells and TUNEL staining was performed to assess cell apoptosis. The results showed that miR-486 mimic could significantly reduce the amount of apoptotic cell in PM2.5-treatment (Figure 1B). In addition, miR-486 mimic also reduced the ratio of Bax/Bcl2 which was determined by western blot (Figure 1C). Taken together, these results demonstrated that the overexpression of miR-486 protected against PM2.5-induced cell apoptosis.
miR-486 suppresses ROS generation in PM2.5-treated human lung alveolar epithelial A549 cells
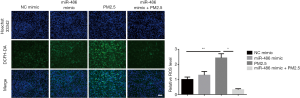
Oxidative stress has been reported to play crucial roles in PM2.5 induced cytotoxicity (12). Then we detected the ROS production after PM2.5 exposure by DCFH-DA fluorescent probe. The results showed that ROS production was increased after PM2.5 treatment. Furthermore, miR-486 mimic administration inhibited ROS production (Figure 2). These data suggested that miR-486 decreased ROS generation in PM2.5-treated human lung alveolar epithelial cells.
miR-486 negatively regulates PTEN and FOXO1
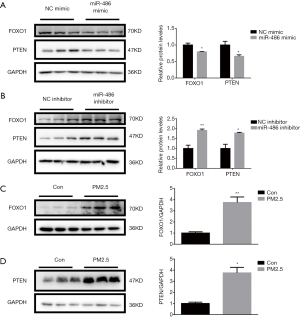
PTEN and FOXO1 were the direct targets of miR-486, which was reported in various cells (13-17). Here, we detected the expression of PTEN and FOXO1 by western blot in miR-486-modified cells. The results showed that miR-486 mimic decreased the protein levels of PTEN and FOXO1, while miR-486 inhibitor increased them in human lung alveolar epithelial A549 cells (Figure 3A,B). Furthermore, the expression of FOXO1 and PTEN was also up-regulated in PM2.5-treated A549 cells, which displayed an inverse correlation with miR-486 expression (Figure 3C,D). These data suggested that miR-486 negatively regulated PTEN and FOXO1 expression, both of which might mediate the protective roles of miR-486 in PM2.5-induced cytotoxicity.
miR-486 decreases PM2.5-induced cell injury by inhibiting PTEN and FOXO1
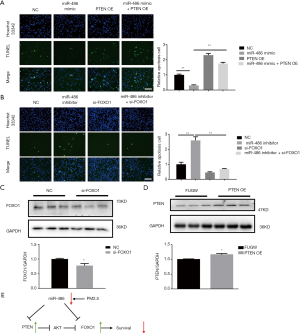
Rescue experiments were performed to investigate the contribution of PTEN and FOXO1 in the protective effects of miR-486 for PM2.5-induced cell injury. In particular, miR-486 mimic transfection decreased the apoptosis of A549 cells after PM2.5 exposure, whereas PTEN overexpression reversed this effect (Figure 4A). Similarly, miR-486 inhibition increased the apoptosis of A549 cells after PM2.5 exposure, whereas FOXO1 knockdown abolished this increase (Figure 4B). Both the overexpression of PTEN and the knockdown of FOXO1 were validated by western blot (Figure 4C,D). Collectively, the results indicated that miR-486 protected PM2.5-induced cell injury by inhibiting PTEN and FOXO1 (Figure 4E).
Discussion
Long-term exposure of PM2.5 increases the occurrence of respiratory diseases. It has been reported that PM2.5 treatment could induce genetic and epigenetic changes in airway tissues. However, the underlying biological processes and molecular mechanisms are still unknown (18). The present work firstly reported the down-regulation of miR-486 after PM2.5 treatment. Moreover, we demonstrated that miR-486 overexpression protected against PM2.5-induced cell injury by targeting PTEN and FOXO1.
MiRNAs are small non-coding RNAs, which act as intracellular regulators in diverse biological processes. Due to their powerful functions and flexible manipulation, miRNAs are becoming the most potential therapeutic tools in pre-clinical research (19). Several studies have reported the correlations between miRNAs and PM2.5 exposure. In an epidemiologic study from Greater Boston area, the authors reported a negative correlation between ambient particles exposure and the expression of miR-1, miR-126, miR-135a, miR-146a, miR-155, miR-21, miR-222 and miR-9 (20). A similar study reported that the higher PM2.5 exposure was negatively associated with several miRNAs, including miR-21-5p, miR-187-3p, miR-146a-5p, miR-1-3p and miR-199a-5p (21). MiRNA profiling are performed to uncover the aberrant miRNAs expression after PM2.5 exposure, but their functions and mechanisms are rarely studied (8,9,18,22-26). Among these differentially expressed miRNAs, only Let-7a, miR-32, miR-1228*, miR-4516 and miR-574-5p were reported to play roles in PM2.5-induced cell injury (9,25,27,28). In this study, we not only reported the protective roles of miR-486 in PM2.5-induced cell injury but also revealed the downstream mediators of miR-486 during this process.
MiR-486 has been reported to play roles in cell growth and cell apoptosis in various cells. For instance, miR-486 reduces H2O2-stimulated cardiomyocyte apoptosis by targeting p53-mediated mitochondrial apoptotic pathway (10). In bovine mammary epithelial cell, the overexpression of miR-486 promotes cell proliferation (15). Human cord blood endothelial colony forming cells (ECFCs) derived miR-486-enriched exosomes protect mice against kidney ischemia/reperfusion injury via targeting PTEN (11). The functions of miR-486 in cancer cells are controversial, some researchers report miR-486 acts as a tumor suppressor and induces cell apoptosis. Others reveal that miR-486 promotes cell proliferation by targeting PTEN (29-32). However, the functional study of miR-486 in PM2.5-stimulated cell injury is unknown. Our work demonstrated that miR-486 mimic administration prohibited cell apoptosis in A549 cells. Further data showed that miR-486 overexpression also decreased PM2.5-stimulated ROS production in A549 cells. Both of which suggested that miR-486 was a potential regulator in preventing PM2.5-stimulated cell injury.
PTEN and FOXO1 been reported to be participated in cell apoptosis regulation. PTEN promotes cell apoptosis by antagonizing the PI3K/Akt signaling pathway (33). FOXO1 promotes cell death by activating the expression of apoptotic proteins (34). As shown in our work, miR-486 negatively regulated the protein levels of PTEN and FOXO1. Moreover, the overexpression of PTEN depressed the anti-apoptotic effects brought by miR-486 overexpression. The knockdown of FOXO1 abolished the pro-apoptotic effects produced by miR-486 inhibition. On the whole, these data demonstrated that miR-486 protected human lung alveolar epithelial A549 cells from PM2.5-stimulated cytotoxicity by targeting PTEN and FOXO1.
Conclusions
In summary, our work found that miR-486 was down-regulated in PM2.5-exposed A549 cells. And miR-486 could relieve cytotoxicity in PM2.5-treated human lung alveolar epithelial A549 cells by targeting PTEN and FOXO1. Our study provided miR-486 administration as a potential therapeutic strategy for PM2.5-induced cell injury.
Acknowledgements
Funding: This study was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-12M-1-006).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ma QY, Huang DY, Zhang HJ, et al. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int Immunopharmacol 2017;50:139-45. [Crossref] [PubMed]
- Klemm RJ, Mason RM, Heilig CM, et al. Is daily mortality associated specifically with fine particles? Data reconstruction and replication of analyses. J Air Waste Manag Assoc 2000;50:1215-22. [Crossref] [PubMed]
- Deng X, Zhang F, Rui W, et al. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol In Vitro 2013;27:1762-70. [Crossref] [PubMed]
- Gualtieri M, Mantecca P, Corvaja V, et al. Winter fine particulate matter from Milan induces morphological and functional alterations in human pulmonary epithelial cells (A549). Toxicol Lett 2009;188:52-62. [Crossref] [PubMed]
- Piao MJ, Ahn MJ, Kang KA, et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch Toxicol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Peng H, Zhao XH, Bi TT, et al. PM2.5 obtained from urban areas in Beijing induces apoptosis by activating nuclear factor-kappa B. Mil Med Res 2017;4:27. [Crossref] [PubMed]
- Pope CA 3rd, Bhatnagar A, McCracken JP, et al. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ Res 2016;119:1204-14. [Crossref] [PubMed]
- Jeong SC, Song MK, Cho Y, et al. Integrative analysis of mRNA and microRNA expression of a human alveolar epithelial cell(A549) exposed to water and organic-soluble extract from particulate matter (PM)2.5. Environ Toxicol 2017;32:302-10. [Crossref] [PubMed]
- Li X, Ding Z, Zhang C, et al. MicroRNA-1228(*) inhibit apoptosis in A549 cells exposed to fine particulate matter. Environ Sci Pollut Res Int 2016;23:10103-13. [Crossref] [PubMed]
- Sun Y, Su Q, Li L, et al. MiR-486 regulates cardiomyocyte apoptosis by p53-mediated BCL-2 associated mitochondrial apoptotic pathway. BMC Cardiovasc Disord 2017;17:119. [Crossref] [PubMed]
- Viñas JL, Burger D, Zimpelmann J, et al. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int 2016;90:1238-50. [Crossref] [PubMed]
- Li J, Zhou Q, Yang T, et al. SGK1 inhibits PM2.5-induced apoptosis and oxidative stress in human lung alveolar epithelial A549cells. Biochem Biophys Res Commun 2018;496:1291-5. [Crossref] [PubMed]
- Wang LS, Li L, Li L, et al. MicroRNA-486 regulates normal erythropoiesis and enhances growth and modulates drug response in CML progenitors. Blood 2015;125:1302-13. [Crossref] [PubMed]
- Xu J, Li R, Workeneh B, et al. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int 2012;82:401-11. [Crossref] [PubMed]
- Li D, Xie X, Wang J, et al. MiR-486 regulates lactation and targets the PTEN gene in cow mammary glands. PLoS One 2015;10:e0118284. [Crossref] [PubMed]
- Ma L, Wang F, Du C, et al. Long non-coding RNA MEG3 functions as a tumour suppressor and has prognostic predictive value in human pancreatic cancer. Oncol Rep 2018;39:1132-40. [PubMed]
- Shao Y, Shen YQ, Li YL, et al. Direct repression of the oncogene CDK4 by the tumor suppressor miR-486-5p in non-small cell lung cancer. Oncotarget 2016;7:34011-21. [Crossref] [PubMed]
- Liu C, Guo H, Cheng X, et al. Exposure to airborne PM2.5 suppresses microRNA expression and deregulates target oncogenes that cause neoplastic transformation in NIH3T3 cells. Oncotarget 2015;6:29428-39. [PubMed]
- Christopher AF, Kaur RP, Kaur G, et al. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect Clin Res 2016;7:68-74. [Crossref] [PubMed]
- Fossati S, Baccarelli A, Zanobetti A, et al. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology 2014;25:68-78. [Crossref] [PubMed]
- Chen R, Li H, Cai J, et al. Fine Particulate Air Pollution and the Expression of microRNAs and Circulating Cytokines Relevant to Inflammation, Coagulation, and Vasoconstriction. Environ Health Perspect 2018;126:017007. [Crossref] [PubMed]
- Duan J, Yu Y, Li Y, et al. Comprehensive understanding of PM2.5 on gene and microRNA expression patterns in zebrafish (Danio rerio) model. Sci Total Environ 2017;586:666-74. [Crossref] [PubMed]
- Hou T, Liao J, Zhang C, et al. Elevated expression of miR-146, miR-139 and miR-340 involved in regulating Th1/Th2 balance with acute exposure of fine particulate matter in mice. Int Immunopharmacol 2018;54:68-77. [Crossref] [PubMed]
- Li X, Lv Y, Hao J, et al. Role of microRNA-4516 involved autophagy associated with exposure to fine particulate matter. Oncotarget 2016;7:45385-97. [PubMed]
- Ku T, Li B, Gao R, et al. NF-kappaB-regulated microRNA-574-5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration. Part Fibre Toxicol 2017;14:34. [Crossref] [PubMed]
- Tsamou M, Vrijens K, Madhloum N, et al. Air pollution-induced placental epigenetic alterations in early life: a candidate miRNA approach. Epigenetics 2018;13:135-46. [Crossref] [PubMed]
- Song L, Li D, Gu Y, et al. Let-7a modulates particulate matter (</= 2.5 mum)-induced oxidative stress and injury in human airway epithelial cells by targeting arginase 2. J Appl Toxicol 2016;36:1302-10. [Crossref] [PubMed]
- Yang D, Ma M, Zhou W, et al. Inhibition of miR-32 activity promoted EMT induced by PM2.5 exposure through the modulation of the Smad1-mediated signaling pathways in lung cancer cells. Chemosphere 2017;184:289-98. [Crossref] [PubMed]
- Yang N, Liang Y, Yang P, et al. Propofol inhibits lung cancer cell viability and induces cell apoptosis by upregulating microRNA-486 expression. Braz J Med Biol Res 2017;50:e5794. [Crossref] [PubMed]
- Gao ZJ, Yuan WD, Yuan JQ, et al. miR-486-5p functions as an oncogene by targeting PTEN in non-small cell lung cancer. Pathol Res Pract 2018;214:700-5. [Crossref] [PubMed]
- Borzi C, Calzolari L, Centonze G, et al. mir-660-p53-mir-486 Network: A New Key Regulatory Pathway in Lung Tumorigenesis. Int J Mol Sci 2017;18. [Crossref] [PubMed]
- Peng Y, Dai Y, Hitchcock C, et al. Insulin growth factor signaling is regulated by microRNA-486, an underexpressed microRNA in lung cancer. Proc Natl Acad Sci U S A 2013;110:15043-8. [Crossref] [PubMed]
- Zhu Y, Hoell P, Ahlemeyer B, et al. PTEN: a crucial mediator of mitochondria-dependent apoptosis. Apoptosis 2006;11:197-207. [Crossref] [PubMed]
- Lu H, Huang H. FOXO1: a potential target for human diseases. Curr Drug Targets 2011;12:1235-44. [Crossref] [PubMed]


