Surgery for intrathoracic tracheoesophageal and bronchoesophageal fistula
Introduction
Tracheoesophageal fistula (TEF) results from an abnormal communication between the posterior wall of the trachea or bronchi and the adjacent anterior wall of the esophagus. It can be acquired or congenital. The onset of the TEF has a negative impact on the patient’s health status and quality of life because of the swallowing difficulties, recurrent aspiration pneumonia, and severe weight loss (1).
According to its location, TEF can be in cervical trachea, in the cervicothoracic transition or mediastinal trachea, which is also denominated as intrathoracic TEF. Regardless of its cause and location, TEF always poses a complex management problem. As opposed to the cervical and cervicothoracic TEF that are frequently associated with prolonged intubation and tracheostomy, the intrathoracic TEF usually results from intrathoracic malignancies, mediastinal infectious lymphadenopathy, surgical and endoscopic manipulation of the mediastinal structures or congenital causes.
The diagnosis and treatment of TEF must follow the same guidelines regardless of its location. The assessment of the patient includes location, extension as well as detection and treatment of concomitant acute or chronic respiratory infection and the patient’s nutritional status.
The definitive treatment is surgical and must be considered in light of several factors, such as the coexistence of tracheal stenosis and the impact of comorbidities in order to determine the feasibility of surgical treatment, as well as its safety and adequacy for the patient.
The surgical approach is planned according to the location of the TEF. Cervical and cervical-mediastinal FTE are best approached via cervicotomy with or without a median sternotomy. The mediastinal or intrathoracic TEF usually requires a right thoracotomy or a video-assisted thoracoscopic surgery (VATS) approach or less often a median sternotomy.
The preparation for surgery is very important and has impact on the outcome. It may take weeks or months and must include weaning from mechanical ventilation, adequate enteral feeding support for the correction of the nutritional disturbances often found in the TEF patient (2).
This chapter focuses on the pathophysiology, diagnosis and management of intrathoracic TEF.
Pathophysiology
There are several acquired conditions causing TEF. The most frequent is prolonged orotracheal intubation (75% of the cases) in which there is an erosion of the tracheal and esophageal wall by the continuous pressure between the endotracheal tube and the esophageal wall, particularly in the presence of a nasogastric or feeding tube within the esophageal lumen. TEF caused by endotracheal tube also depend on the pressure exerted by the cuff, where pressures in excess of 30 mmHg can significantly reduce mucosal perfusion and result in focal tracheal necrosis. Post-intubation TEF has been reported in 0.3% to 3% of patients submitted to mechanical ventilation. Concurrent factors such as the patient’s nutritional status, steroid use and the presence of infection can ultimately predispose to development of a TEF (2-4).
Other causes include tracheostomy, trauma, malignancy, esophageal perforation, presence of foreign body, mediastinitis, tracheal resection, surgery of the esophagus, mediastinum, the presence of mediastinal lymphadenopathy, and infections. Traumatic TEF can occur after blunt or open chest trauma. In blunt chest trauma, the TEF is usually and located at the level of the carina. The TEF often becomes clinically detectable a few days later because of tracheal wall necrosis. In open chest trauma the perforation site will determine the TEF location. In tracheostomy patients the TEF may ensue as a result of inadequate positioning of the tracheostomy. In such instances, posterior dislocation of the tip or the balloon of the cannula will exert pressure in the posterior tracheal wall against the esophagus, resulting in a TEF (2,3).
Diagnosis
Symptoms and signs of a TEF may vary widely, depending on the etiology and clinical setting. A high index of suspicion is therefore required in order to detect a TEF.
The clinical presentation will vary from symptoms and signs directly attributable to the fistula itself, as well as to complications arising from the fistula, particularly pulmonary and from the underlying disease.
Persistent cough during oral feeding (Ono’s sign) is highly suggestive of a communication between the esophagus and airway. Patients may expectorate sputum mixed with food. Other symptoms include an increase in tracheal secretions, dysphagia and gagging during feeding. Such symptoms are worsened by certain positions, decubitus, and when swallowing liquids. The onset of recurrent pneumonia and progressive weight loss are the hallmark of TEF (5).
In patients under mechanical ventilation and orotracheal intubation, TEF is suspected based on the increase of tracheobronchial secretions, the suction of gastric contents from the airway associated with sudden gastric and abdominal distention due to “ventilation” of the esophagus and stomach (3,5).
Radiologic and endoscopic examination
Diagnostic exams include computed tomography, bronchoscopy, upper gastrointestinal endoscopy, and contrast examination of the esophagus. A chest radiograph may initially be normal, but will subsequently demonstrate the pulmonary complications attributable to the fistula, that include pulmonary infiltrates, pneumonia and ARDS. Other non-specific findings may suggest the diagnosis, such as the presence air distension of the esophagus.
High resolution multislice computed tomography (HRCT) is able to diagnose the TEF by means of the demonstration of the fistulous tract itself. By the same token, the HRCT scan enables a thorough assessment of the pulmonary parenchyma and mediastinum. Despite the advantages and the excellent imaging quality provided by the HRCT, contrast radiography (barium swallow) and the fiberoptic upper gastrointestinal and airway endoscopy remain the mainstay for the diagnosis of TEF worldwide (5).
Endoscopic examination of the upper aerodigestive tract remains the most useful diagnostic tool to detect and evaluate patients with TEF. Bronchoscopy and esophagoscopy may be performed using rigid or flexible instrumentation, and under general or local anesthesia with sedation, as determined by the clinical setting (5).
The flexible bronchoscopy offers a direct visualization of the TEF, pinpointing its location and extent of the fistula. The exact location is measured using the distance of the anatomical reference points, such as the vocal folds in the proximal fistulas, and the tracheal carina in the distal fistulas. Biopsies of the posterior membranous trachea adjacent to the fistula are taken in order to obtain a histologic diagnosis if malignancy or tuberculous origin are suspected. The distal tracheobronchial tree is visualized and bronchial washings are obtained and sent for culture to identify microorganisms as a guide to the systemic antibiotic therapy (1,3,5).
Upper gastrointestinal endoscopy helps assessing the TEF, particularly in regards to its extent that can differ between the esophageal and bronchial side. Esophagoscopy can easily visualize a large fistula; nevertheless it is less accurate in detecting small fistulas, which may be hidden within the folds of esophageal mucosa.
In an intubated and sedated patient, ideally both esophagoscopy and bronchoscopy are carried out simultaneously. The entire trachea is then viewed by simply withdrawing the endotracheal tube leaving its tip at the level of the vocal cords. Meanwhile, the esophagus is insufflated with air either by an esophagoscope or by a nasogastric tube is advanced into the esophagus. This maneuver will create a bulging of the esophagus that provides an excellent view of the real dimension of the TEF by the bronchoscopy observer (1,3,5).
In summary, the diagnosis of TEF must be suspected based on clinical signs, symptoms and confirmed by imaging, preferably using CT scan or a contrast esophagram. Airway and upper gastrointestinal endoscopy will provide the exact location and extension of the TEF that is an important step towards planning the management strategy.
Pre-operative evaluation and infection control
All patients are evaluated at the outpatient clinic. It is of utmost importance that patients are weaned from mechanical ventilator support. Assessment includes computed tomography scan of the larynx, trachea, and lungs; flexible or rigid bronchoscopy or both; and upper gastrointestinal endoscopy in all patients. Recurrent pulmonary infections and aspiration control are dealt with physical therapy, cessation of all oral intake, and antibiotics. Enteral nutritional support is ascertained preferably through a percutaneous endoscopic gastrostomy or jejunostomy, if necessary. Routine consultations with the nutrition department are scheduled.
Patients with TEFs larger than 10 mm and recurrent pulmonary infections should undergo a preoperative tracheostomy placed at the fistula site, or as close to it as possible, to minimize tracheal damage. The procedures are guided by bronchoscopy, and the cuff of the cannula is placed distal to the esophageal defect.
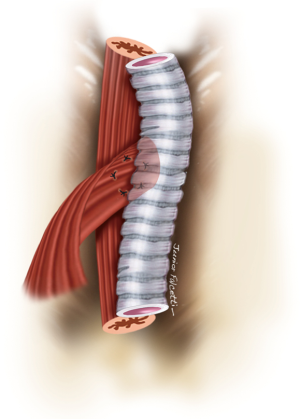
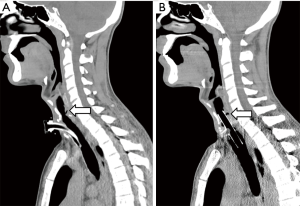
Endoluminal tracheal or esophageal stents may have a limited temporizing role to play in controlling ongoing pulmonary soilage until definitive surgical treatment can be undertaken, but they are unlikely to provide satisfactory definitive management of benign acquired TEFs (6). Self-expanding esophageal metallic stents should be avoided. They are difficult to remove, prone to enlarge the esophageal defect and might not prevent pulmonary soilage (Figure 1). If necessary, we prefer to use silicone tracheal stents in order to control pulmonary secretions. They are easier to place and to remove. Furthermore, in cases associated with tracheal stenosis, phonation and nasal breathing can be restored (Figure 2).
Anesthesia induction
Anesthesia for TEF procedures should follow the same principles applied for tracheal resection surgery. Preoperative endoscopic examination must be performed in order to check for fistula location and size. We routinely do a flexible and/or rigid bronchoscopy at the operating room prior to the operation (1). Patients often have a tracheostomy located distal to the TEF, or a tracheal device that secures the airway. Thus, anesthesia induction and ventilation can be safely performed. Neuromuscular blockers can be safely used after adequate ventilation is achieved with Propofol (1.5–2.5 mg/Kg IV).
Individuals with TEF without tracheostomy or other tracheal devices should prompt care during anesthesia induction. Small fistulas (≤5 mm) are unlikely to affect ventilation. However, large fistulas (≥10 mm) can have a serious impact on ventilation (7). In severe cases, ventilation with positive pressure might not be possible at all. Thus, in those cases, we recommend to start the procedure with moderate sedation and to perform a flexible bronchoscopy under topical anesthesia (lidocaine 1%) (8).
Endotracheal intubation under direct endoscopic vision is then performed. The cuff of the endotracheal tube should be placed below the TEF. After securing the airway, propofol and neuromuscular blocking agents can be safely used.
In the setting of a large fistula, airway management must not be without endoscopic guidance. Endotracheal and tracheostomy tubes can be misplaced in the esophagus, leading to worsening of the esophageal defect. Furthermore, esophageal intubation might not be detected immediately, causing desaturation and unnecessary emergency measures.
Surgical technique
Surgical repair of a TEF should be an elective procedure (1,6). It is widespread consensus that mechanical ventilation is an absolute contraindication to surgical repair (1,3,6,9-11). Respiratory infections must be treated with antibiotics and, in patients with persistent respiratory soilage, draining gastrostomy and feeding jejunostomy can be performed (12).
Most fistulas are located in in the upper and mid esophagus/trachea and can be operated on through a low cervical collar incision. If the TEF is distally located, a partial sternotomy can improve exposure of the carina (3) (Figure 3). Fistulas that are located at the carina, or very distal in the trachea, should be approached through a right thoracotomy in the fourth intercostal space. Accordingly, bronchoesophageal fistulas should be operated on through a thoracotomy on the affected side.
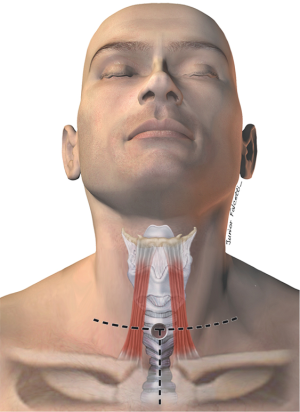
At our institution, the surgical approach is dictated upon the following criteria: (I) location of the fistula; (II) concomitant tracheal stenosis; (III) size of the TEF (Figure 4).
TEF less than 5 mm in diameter without tracheal stenosis
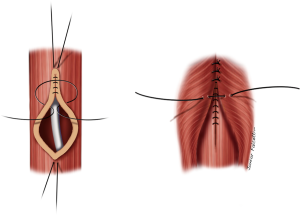
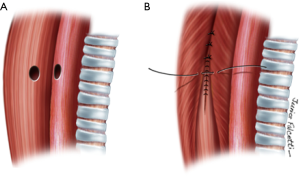
Small fistulas (≤5 mm) without tracheal stenosis can be approached by a lateral cervicotomy, without the need for a tracheal resection. The trachea and the esophagus are dissected, and the recurrent laryngeal nerve is identified. Care should be taken with this approach, since patients often have tracheostomy or previous operations. Thus, intense scarring might increase the odds of laryngeal nerve injury (3,11). After dissection of the fistula, it is divided and sutured (Figure 5). The tracheal defect is closed with interrupted, absorbable sutures (polydioxanone 4-0). The esophagus is repaired in two layers (Figure 6). Vascularized muscle flaps are interposed between the airway and esophagus (Figure 7). Strap muscles are usually sufficient to buttress the repairs and separate the suture lines, thus minimizing the chance of fistula recurrence.
Another option for small fistulas with normal trachea is to perform a “trans-tracheal” approach, i.e., an anterior incision of the trachea at the site of the TEF, without a formal tracheal resection. In this setting, after the fistula is located, the tracheal and esophageal walls are separated with sharp dissection, followed by a circumferential dissection of the distal and proximal trachea (1,9,13,14). The esophagus is closed in the same manner as described, and the repair is buttressed with a muscle flap. Since the airway is not resected, an end-to-end anastomosis can be performed with minimal tension.
We have used this technique in five cases. All of them had uneventful recoveries (1). Other authors have also reported excellent results with this technique (11,13,14). Even though the trachea is incised and a primary anastomosis is performed, post-operative tracheal stenosis does not seem to be an issue. Furthermore, dissection is kept far from the tracheoesophageal groove, leading to less strain on the recurrent laryngeal nerve.
TEF larger than 10 mm in diameter with or without tracheal stenosis
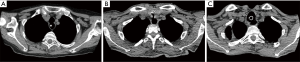
Large lesions (≥10 mm) usually require concomitant tracheal resection and reconstruction for repair of the underlying TEF. Often, the defect in the membranous wall is so large that it cannot be simply sutured. In this setting, the distal trachea is transected below the TEF or tracheal stenosis. A cuffed endotracheal tube is placed into the distal trachea and ventilation is performed across the operative field (3). This allows excellent exposure of the esophagus and the TEF (Figure 8). The proximal trachea is transected above the area of disease or stenosis. The extent of trachea resected should be limited to a minimum in order to minimize anastomotic tension.
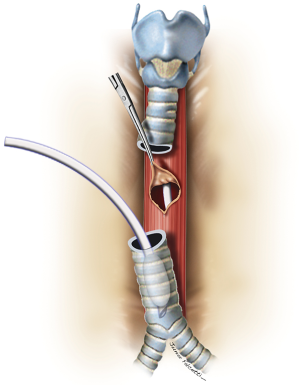
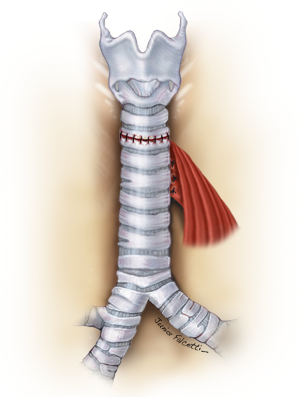
The esophageal mucosal edges are debrided and the defect is closed over a nasogastric tube in two layers, as previously shown (Figure 6). A pedicled strap muscle flap is mobilized and placed over the esophageal repair to prevent recurrent fistulization (3) (Figure 9). Tracheal anastomosis is performed with a continuous running suture of polydioxanone 4-0 (PDS II; Ethicon, Bridgewater, NJ) in the membranous wall and separated sutures of polyglactin 3-0 (Vicryl, Ethicon, Bridgewater, NJ) in the cartilaginous wall (15) (Figure 10). Laryngotracheal reconstruction, if necessary, is performed according to the technique described by Pearson et al. (16) and Grillo et al. (17). Suprahyoid laryngeal release maneuvers can be used according to the surgeon’s judgment in order to reduce anastomosis tension (15,18).
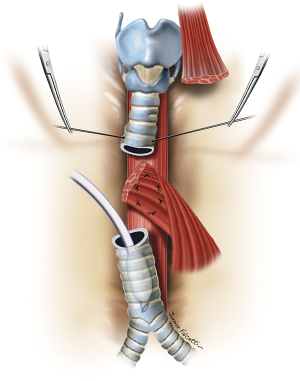
TEF of any diameter, with tracheal stenosis
Fistulas associated with tracheal stenosis will necessarily require a primary tracheal resection with end-to-end anastomosis. Care should be taken in patients with TEFs associated with long segment stenosis (≥4 cm). In those cases, resection of the full extent of the trachea might lead to increased tension in the anastomosis, dehiscence and recurrent stenosis and TEF (3,9,10). If the tracheal stenosis is deemed non-resectable, the TEF can be approached by incision of the anterior wall of the trachea at the site of the fistula. The esophageal defect is closed, and tracheal reconstruction accomplished with a tracheostomy or preferably, over a silicone T-Tube (1,15). It restores airway patency and phonation while a proper healing of the underlying anastomosis takes place (19,20). Long-term silicone stenting after surgery in this scenario yields to good a surgical outcome, and removal of the T-Tube should be attempted after 6 to 12 months (20-22).
Post-operative management
Patients are sent to the intensive care unit for the first 24–48 hours and afterwards discharged to the general ward. Between the 5th and 7th postoperative day a barium swallow is performed to check for esophageal leaks before oral intake is reinitiated. Patients are discharged when adequate feeding is resumed either by oral intake or by feeding tube in case of dysphagia/aspiration or both. Patients are seen at the outpatient thoracic surgical clinic at 2 weeks; 1, 3, and 6 months after hospital discharge; and twice yearly thereafter (1). Flexible bronchoscopy and upper endoscopy are routinely done 3 months after the surgical procedure. In the event of any complaints related to the operation or clinical signs or symptoms of complications, imaging studies, flexible bronchoscopy, and upper gastrointestinal endoscopy are performed (1).
Post-operative complications
Surgery for TEF is challenging and its morbidity rate is not negligible. In fact, the complication rate has been reported to be between 32% and 56%, mainly anastomotic issues or fistula recurrences (14). Since benign acquired TEF is an uncommon condition, reported complications following this type of operation are limited to a few retrospective case series from large referral centers for airway diseases.
The largest experience was published by the Muniappan et al. (9) and refers to a 2-period cases series from the Massachusetts General Hospital, In Boston. The first period encompasses 38 patients that were operated on from 1975 to 1991, and in the second period, 36 additional patients (1992–2010). The most common causes were post-intubation injury (47%), trauma (17%), prior laryngectomy (17%), and prior esophagectomy (11%). Twenty patients (56%) experienced postoperative morbidity. Six patients (17%) required tracheostomy or T-tube placement for pneumonia, airway obstruction, or partial anastomotic dehiscence. TEF recurred in four patients (11%). Pneumonia and wound infection each occurred in three patients (8%). Mortality was 10.5% in the first period, and 2.8% in the second period. This drop in mortality certainly reflects the institution’s excellence as a referral center, and progression of preoperative and postoperative care. As reported, 13 patients (36%) were referred to the institution after failed initial repair of the TEF, a proportion twice higher than the reported in their early experience. However, the authors also noted a five-fold increase in the number of patients requiring tracheal appliance (29%) after operative repair of the TEF compared with the earlier series (6%).
Shen and colleagues at The Mayo Clinic reported their experience in TEF repair in 35 patients, from 1978 to 2007 (10). As opposed to the other series, post-intubation lesions accounted for only 5.7% of cases. Most cases occurred after prior esophageal surgery (31.4%), Laryngotracheal trauma (17.1%) and granulomatous mediastinal infections (14.3%). In this series, 26% were located in the distal trachea, whereas 40% were actually bronchoesophageal fistulas. Nineteen patients (54.3%) had postoperative complications and 8 patients (22.8%) required reoperation for complications. Respiratory failure (17.1%), pneumonia (14.3%), esophageal leak (11.4%) and postoperative bleeding requiring reoperation (11.4%) were the most common complications. Operative mortality was 5.7% and three patients (8.6%) developed recurrent TEF.
Our experience at the University of São Paulo, Brazil, was reported in 2016 (1). It consisted of 20 patients that were submitted to TEF repair within a 10-year period. The most common cause of TEF was post-intubation injury (80%). Complications occurred in 11 patients (55%), and the most common were subcutaneous emphysema (20%) and pneumonia (20%). TEF recurrence was found in one patient (5%) that had two prior attempts of TEF repair at another center. This same patient also had a dehiscence of the tracheal anastomosis. Three individuals (15%) had recurrent tracheal stenosis after the primary repair. They were treated with silicone T-Tubes. One T-tube was removed 18 months after the initial operation. The two remaining patients still have indwelling silicone stents, but are able to breathe normally and resumed oral intake.
One postoperative death occurred in our series (5%). It consisted of a TEF associated with a 4-cm tracheal stenosis that required resection of the anterior portion of the cricoid cartilage. Deep venous thrombosis and pulmonary embolism occurred in the early postoperative period. Pneumonia and pulmonary sepsis followed; the patient was intubated 45 days after the operation and died at the 60th postoperative day.
Complication rates after the operative management TEFs vary widely across different institutions, and are dependent on the expertise and referral patterns of each center (Table 1). Nevertheless, high morbidity is the general rule. Fistula recurrence rates of up to 11% have been reported, with mortality ranging from 0–10% (1,9-12,23-26).
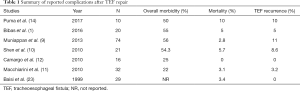
Full table
Results
TEF repair is a complex procedure, and as stated earlier, high morbidity and mortality are expected. Nonetheless, surgical management yields excellent long-term results, and it should be considered the first-line treatment for this condition. Definitive fistula closure occurs in about 90–95% of the cases (1,9-12,23-26). Although there are no published predictors for TEF recurrence, previous attempted repair, prior esophagectomy, and laryngectomy seem to be associated with poor outcomes (9,10). In the report by Muniappan and colleagues (9), 13 patients had failed initial surgical or endoscopic repairs; one fistula recurred and could not be closed. Furthermore, other series have reported complications and fistula recurrence after prior failed operations, often requiring a permanent tracheal appliance (1,10,14). In our cohort, 3 patients had failed prior TEF repairs (1). Only one individual had an uneventful recovery. The other two have indwelling tracheal stents.
However, individuals with initial failed repairs of TEF should not be denied surgical procedures (1). Camargo and colleagues (12) operated on three cases of recurrent TEF, and only one patient experienced a small tracheal dehiscence. All three reported cases had good long-term functional outcomes. Similar data have been reported by Altorjay and colleagues (24). In a 10-year series, the group treated eight patients with recurrent TEF; one patient had been operated on four times. All TEFs were successfully repaired.
Conclusions
TEF repair is a challenging and demanding procedure. Careful selection of patients and optimization of clinical status is of utmost importance for a successful repair. This encompasses weaning from mechanical ventilation and enteral nutritional support, as well as adequate control of respiratory infections. Single-stage primary repair of both airway and esophagus is the treatment of choice, and provides the best long-term results. If present, tracheal stenosis should be resected and reconstruction accomplished by means of an end-to-end primary anastomosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bibas BJ, Guerreiro Cardoso PF, Minamoto H, et al. Surgical Management of Benign Acquired Tracheoesophageal Fistulas: A Ten-Year Experience. Ann Thorac Surg 2016;102:1081-7. [Crossref] [PubMed]
- Couraud L, Ballester M, Delaisement C. Acquired tracheoesophageal fistula and its management. Semin Thorac Cardiovasc Surg 1996;8:392-9. [PubMed]
- Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am 2003;13:271-89. [Crossref] [PubMed]
- Rathinam S, Kanagavel M, Tiruvadanan B, et al. Dysphagia due to tuberculosis. Eur J Cardiothorac Surg 2006;30:833-6. [Crossref] [PubMed]
- Chauhan SS, Long J. Management of Tracheoesophageal Fistulas in Adults. Curr Treat Options Gastroenterol 2004;7:31-40. [Crossref] [PubMed]
- Shen KR. Management of acquired nonmalignant tracheoesophageal fistula: Surgical pearls. J Thorac Cardiovasc Surg 2017;154:e123. [PubMed]
- Echeverri M, Martín Herrero J, Vicente R, et al. Consideraciones anestésicas en una paciente con fístula traqueoesofágica adquirida. Rev Esp Anestesiol Reanim 2008;55:584-6. [Crossref] [PubMed]
- Jose RJ, Shaefi S, Navani N. Sedation for flexible bronchoscopy: current and emerging evidence. Eur Respir Rev 2013;22:106-16. [Crossref] [PubMed]
- Muniappan A, Wain JC, Wright CD, et al. Surgical treatment of nonmalignant tracheoesophageal fistula: A thirty-five year experience. Ann Thorac Surg 2013;95:1141-6. [Crossref] [PubMed]
- Shen KR, Allen MS, Cassivi SD, et al. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg 2010;90:914-8. [Crossref] [PubMed]
- Macchiarini P, Verhoye JP, Chapelier A, et al. Evaluation and outcome of different surgical techniques for postintubation tracheoesophageal fistulas. J Thorac Cardiovasc Surg 2000;119:268-76. [Crossref] [PubMed]
- Camargo JJ, Machuca TN, Camargo SM, et al. Surgical treatment of benign tracheo-oesophageal fistulas with tracheal resection and oesophageal primary closure: is the muscle flap really necessary? Eur J Cardiothorac Surg 2010;37:576-80. [Crossref] [PubMed]
- Liu J, Wu W, Liu S, et al. A modified tracheal transaction approach for the repair of nonmalignant tracheoesophageal fistulas: a report of 5 cases. ORL J Otorhinolaryngol Relat Spec 2017;79:147-52. [Crossref] [PubMed]
- Puma F, Vannucci J, Santoprete S, et al. Surgery and perioperative management for post-intubation tracheoesophageal fistula: Case series analysis. J Thorac Dis 2017;9:278-86. [Crossref] [PubMed]
- Bibas BJ, Terra RM, Oliveira AL Junior, et al. Predictors for postoperative complications after tracheal resection. Ann Thorac Surg 2014;98:277-82. [Crossref] [PubMed]
- Pearson FG, Brito-Filomeno L, Cooper JD. Experience with partial cricoid resection and thyrotracheal anastomosis. Ann Otol Rhinol Laryngol 1986;95:582-5. [Crossref] [PubMed]
- Grillo HC, Mathisen DJ, Wain JC. Laryngotracheal resection and reconstruction for subglottic stenosis. Ann Thorac Surg 1992;53:54-63. [Crossref] [PubMed]
- Terra RM, Minamoto H, Carneiro F, et al. Laryngeal split and rib cartilage interpositional grafting: Treatment option for glottic/subglottic stenosis in adults. J Thorac Cardiovasc Surg 2009;137:818-23. [Crossref] [PubMed]
- Bibas BJ, Bibas RA. A new technique for T-tube insertion in tracheal stenosis located above the tracheal stoma. Ann Thorac Surg 2005;80:2387-9. [Crossref] [PubMed]
- Terra RM, Bibas BJ, Minamoto H, et al. Decannulation in tracheal stenosis deemed inoperable is possible after long-term airway stenting. Ann Thorac Surg 2013;95:440-4. [Crossref] [PubMed]
- Sihag S, Wright CD. Prevention and Management of Complications Following Tracheal Resection. Thorac Surg Clin 2015;25:499-508. [Crossref] [PubMed]
- Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: Prognostic factors and management. J Thorac Cardiovasc Surg 2004;128:731-9. [Crossref] [PubMed]
- Baisi A, Bonavina L, Narne S, et al. Benign tracheoesophageal fistula: results of surgical therapy. Dis Esophagus 1999;12:209-11. [Crossref] [PubMed]
- Altorjay Á, Mucs M, Rüll M, et al. Recurrent, Nonmalignant Tracheoesophageal Fistulas and the Need for Surgical Improvisation. Ann Thorac Surg 2010;89:1789-96. [Crossref] [PubMed]
- Fiala P, Cernohorsky S, Cermák J, et al. Tracheal stenosis complicated with tracheoesophageal fistula. Eur J Cardiothorac Surg 2004;25:127-30. [Crossref] [PubMed]
- Cardoso PFG, Bibas BJ, Minamoto H, et al. Prophylaxis and Treatment of Complications After Tracheal Resection. Thorac Surg Clin 2018;28:227-41. [Crossref] [PubMed]