Prediction of fluid responsiveness in ventilated patients
Introduction
Except in rare cases such as in cardiogenic shock, hypovolemia is present in patients with acute circulatory failure so that fluid administration should be performed in the early resuscitation phase of shock states, in particular in cases of sepsis. In this regard, some experts recommend to infuse a fixed volume of crystalloids (30 mL/kg within the first 3 hours) as soon as the diagnosis of septic shock is made (1), although others claim a more individualized fluid management strategy (2,3). The main goal of fluid administration is to correct hypovolemia and thus to increase the venous return, the cardiac preload, and ultimately the cardiac output and oxygen delivery. Nevertheless, if the initial volume resuscitation is insufficient to completely correct tissue hypoxia, continuing fluid administration often raises a therapeutic dilemma. On the one hand, hemodynamic benefits can be still expected from a complete resolution of hypovolemia. On the other hand, not all patients are fluid responsive at this stage (4), and it is now widely recognized that fluid overload results in tissue edema, delays weaning from mechanical ventilation and is an independent predictor of mortality in critically ill patients (5), in particular in septic patients (6-8) and those with acute respiratory distress syndrome (ARDS) (9). Thus, at this stage, it is crucial for physicians to be able to reliably predict fluid responsiveness (3). For this purpose, several tests have been used for many years (10). Historically, static markers of cardiac preload, such as the central venous pressure (CVP) or the pulmonary artery occlusion pressure (PAOP) have been used. More recently, some dynamic tests have been developed. Most of them are based on heart-lung interactions during mechanical ventilation. In this review, we will first summarize which tests could be helpful to predict fluid responsiveness in ventilated patients and second how the concept of fluid responsiveness could be integrated at bedside.
Static markers of cardiac preload
CVP and PAOP are the most commonly used markers of right and left cardiac ventricular preload, respectively. It is now well admitted that both variables could not reliably predict fluid responsiveness (10,11). This poor reliability mainly results from the fact that a same value of CVP or PAOP could correspond to a preload responsiveness state or a preload unresponsiveness state, depending on the slope of the Frank-Starling curve, which varies among patients in function of the systolic cardiac function. This poor reliability of CVP and PAOP to predict fluid responsiveness might also be explained by the fact that the values of CVP and PAOP also depend on the transmission of the pleural pressure to the cardiac structures, which is a confounding factor. Thus, for a same value of CVP or PAOP, fluid administration could result in a small or a large increase in stroke volume and cardiac output. In spite of this poor reliability for predicting fluid responsiveness, the CVP is still relatively widely used for this purpose, as suggested by the FENICE study (12). Other static markers of cardiac preload such as the global end-diastolic volume obtained by transpulmonary thermodilution, the left end-diastolic area or volume measured by echocardiography or the flow time of aortic blood flow measured by esophageal Doppler share the same limitation (10,11).
Very recently, the reliability of the end-expiratory inferior vena cava diameter, a static variable that could be a good estimate of the transmural right atrial pressure and hence of cardiac preload, has also been investigated (13). The end-expiratory inferior vena cava diameter was not obtained in 22% of patients, whereas the echocardiography examination was performed by experienced operators, and could predict fluid responsiveness or unresponsiveness in only 29% of the ventilated patients with a specificity of 80%. The reliability was even poorer in patients with an elevated intra-abdominal pressure (>12 mmHg). Nevertheless, a value of end-expiratory inferior vena cava diameter which was very low (≤8 mm) or very high (≥28 mm) could predict fluid responsiveness and fluid unresponsiveness, respectively, with a specificity of 95% (13).
Dynamic tests based on heart-lung interactions (Table 1)
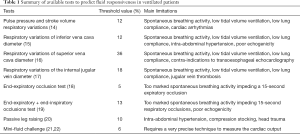
Full table
Physiology of heart-lung interactions
Most of the dynamic tests developed to predict fluid responsiveness in ventilated patients are based on heart-lung interactions. The physiological background lies on the cyclic changes in the loading conditions of the right and left ventricles induced by the positive pressure mechanical ventilation. In brief, the idea behind is that the more the stroke volume changes during the mechanical ventilation cycle, the more likely the patient’s heart is preload responsive (23).
Pulse pressure and stroke volume respiratory variations
The pulse pressure (systolic-diastolic arterial pressure) is a surrogate of the stroke volume. In this regard, Michard et al first demonstrated that the pulse pressure respiratory variations (PPV) could reliably predict fluid responsiveness in mechanically ventilated patients with septic shock, with a threshold value of 13% (14). This has been widely confirmed afterwards (24,25). In a recent meta-analysis, PPV could predict fluid responsiveness with a sensitivity of 88%, a specificity of 95% and a threshold value of 12% (25). Nevertheless, some well-known limitations preclude the use of PPV and may induce false-positives or false-negatives (26). PPV is unreliable in cases of open-chest conditions, spontaneous breathing activity, low tidal volume ventilation, high-frequency ventilation, cardiac arrhythmias, intra-abdominal hypertension and in case of low lung compliance (26,27). These limitations contribute to extend a zone of uncertainty of PPV (grey zone) where values between 9% and 13% can be associated with fluid responsiveness as well as fluid unresponsiveness (28). However, it must be stressed that low tidal volume ventilation should not preclude the use of PPV. Myatra et al. recently proposed a new fluid responsiveness test called “the tidal volume challenge” (29). They demonstrated that an increase in the absolute value of PPV ≥3.5% induced by a transient increase in tidal volume from 6 to 8 mL/kg for 1 minute could reliably predict the increase in cardiac output in response to a fluid bolus performed at a tidal volume of 6 mL/kg whereas the PPV value obtained at 6 mL/kg tidal volume was unreliable for this purpose (29). Similar results were found for stroke volume variation (SVV) obtained from a contour analysis cardiac output monitor (threshold value: 2.5%) (29). Thus, using a tidal volume challenge might overcome the limitations of PPV as a predictive index of fluid responsiveness during low tidal volume ventilation (30). Application of a temporary increase in tidal volume (from 8 to 12 mL/kg) has been recently proposed for patients ventilated with normal tidal volume but with PPV values within the grey zone (31). Nevertheless, the applicability of PPV has nowadays decreased in critically ill patients, mainly because of maintenance of spontaneous breathing due to the use of less sedation. In this regard, Preau et al. showed that PPV could be used without any contra-indications in only 17% of cases (32). Of note, the study considered that the low tidal volume was one of these contra-indications, before the tidal volume challenge had been described.
Besides PPV, the ability of many other invasive or non-invasive surrogates of stroke volume to reliably assess SVV has been studied in the last decades. In this regard, the cyclic respiratory variations of the stroke volume estimated from the pulse-contour analysis (33), or from the peak velocity of the Doppler signal in the left ventricular outflow tract at echocardiography (34), from the aortic blood flow obtained with the esophageal Doppler (35) or from the amplitude of the plethysmographic signal (36,37) as well as the PPV estimated non-invasively using the volume-clamp method (33), were shown to be all reliable predictors of fluid responsiveness. Obviously, all these indices have the same limitations as PPV.
Respiratory variations of the vena cava diameter
The respiratory variations of the inferior vena cava diameter have been reported to reliably predict fluid responsiveness in ventilated patients (15,38,39), although more recent studies found divergent results in mixed populations of surgical and medical patients in shock (40,41). The reasons for these discrepancies have not been elucidated, but the role of increased abdominal pressure in one of these studies cannot be excluded (41). Zhang et al. reported in a meta-analysis pooling eight studies involving 235 patients that the pooled sensitivity and specificity were 76% and 86% respectively with an average area under the receiver operating characteristic curve of 0.84 (42).
The diameter of the inferior vena cava must be measured using transthoracic echocardiography in the subcostal longitudinal long-axis view of the vessel in M-mode, approximately 2 cm from the junction with the right atrium and usually upstream to the supra-hepatic vein inlet (43,44). Importantly, since it is based on heart-lung interactions, the respiratory variations of the inferior vena cava diameter suffer from most of the limitations of PPV and SVV. In this regard, ten situations in which the respiratory variations of the inferior vena cava fail to accurately predict fluid responsiveness were proposed (45). In particular, patients must be fully adapted to ventilator and the presence of intra-abdominal hypertension might also induce some false positives or negatives. Unlike PPV or SVV, the respiratory variations of the inferior vena cava diameter can be used in patients with cardiac arrhythmias.
In a study including 66 septic shock patients, Vieillard-Baron et al. reported that the respiratory variations of the superior vena cava diameter—called “collapsibility” of the superior vena cava—reliably predicted fluid responsiveness in mechanically ventilated patients (16). The measurement of the superior vena cava diameter requires transoesophageal echocardiography. However, since the publication of this pilot study, divergent results were reported (40,41). In particular, a recent multicenter and prospective study including 540 ventilated patients with various types of shock showed that the respiratory variations of the superior vena cava diameter had the highest specificity for detecting preload dependence (41).
Respiratory variations of the internal jugular vein diameter
The distensibility of the internal jugular vein was also proposed to predict fluid responsiveness in mechanically ventilated patients with sepsis (17) as well as after cardiac surgery (46), with a threshold value varying from 13% (46) to 18% (17). The reliability of the distensibility of the internal jugular vein to predict fluid responsiveness was shown to be similar to PPV (17) and to the distensibility of the inferior vena cava (46). Interestingly, combining the distensibility of the internal jugular vein and PPV markedly increased the reliability of these predictive indices (17). As the distensibility of the internal jugular vein is also based on heart-lung interaction, this index should share with PPV its main limitations.
Unlike the measurement the inferior vena cava diameter (41), the measurement of the internal jugular vein short-axis diameter seems to be easy to obtain using the M-mode with the ultrasound probe placed perpendicular to the skin at the level of the cricoid cartilage (17,46).
End-expiratory occlusion test
In mechanically ventilated patients, each insufflation increases the intrathoracic pressure and impedes the venous return. Interrupting mechanical ventilation at end-expiration (i.e., just before the next insufflation) for a few seconds should thus prevent the cycling decrease in venous return and cardiac preload, which increase transiently. This “preload challenge” results in a significant increase in cardiac output if the patient’s heart is preload responsive. In this regard, it was previously shown that an increase in cardiac output ≥5% during an end-expiratory occlusion of 15 seconds could be used to reliably predict fluid responsiveness in critically ill patients, including patients with cardiac arrhythmias and spontaneous breathing (18,27,33,47). The predictive accuracy of the test is not modified in patients with acute respiratory syndrome by a level of positive end-expiratory pressure varying from 5 to 15 cmH2O (47). Thus, the main limitation of the end-expiratory occlusion test is a too marked spontaneous breathing activity, impeding a 15 seconds expiratory occlusion. It must be noted that because of the low threshold value, the cardiac output must be measured by a very precise and accurate method. In this regard, in all the previous studies (18,27,33,47), the response to the end-expiratory occlusion was assessed using a pulse contour analysis-derived cardiac output, which most often requires an invasive haemodynamic monitoring. The precision of this technique is sufficiently acceptable to detect changes in cardiac output by 5% (48).
Interestingly, our group (19) and others (49) have recently demonstrated that the end-expiratory occlusion test could be used even in patients without invasive haemodynamic monitoring, by using the echocardiography to measure the cardiac output. An increase ≥5% (19) or ≥9% (49) in the velocity-time integral of the left ventricular outflow tract induced by a 15- or a 12-second expiratory hold could reliably predict fluid responsiveness. Nevertheless, the threshold value of 5% was very close to the intra-observer variability for the velocity-time integral measurement (19,21,50,51). Similarly, the threshold value of 9% was below the least significant change found by Georges and colleagues for the velocity-time integral measurement (49). In other words, the prediction of fluid responsiveness from the changes in velocity-time integral during an end-expiratory occlusion might be limited by the precision of echocardiography. Nevertheless, we found that a decrease in velocity-time integral ≥8% during an end-inspiratory occlusion also allowed the prediction of fluid responsiveness (19). When considering the added effects in absolute values of end-inspiratory and end-expiratory occlusions on the velocity-time integral, the predictive accuracy for predicting fluid responsiveness was similar to that of the only end-expiratory occlusion (19). However, the diagnostic threshold increased to 13% (19) which is more compatible with the precision of echocardiography (19,21,50,51).
Passive leg raising
The passive leg raising could be considered as an “internal preload challenge” (10). Boulain et al. showed that this postural maneuver resulted in an increase in both right and left ventricular preload (52). Interestingly, the changes in stroke volume induced by the passive leg raising and by a fluid challenge were strongly correlated, suggesting that the passive leg raising might mimic the hemodynamic effects of an about 300 mL fluid challenge (52). Thereafter, it was demonstrated that fluid responsiveness could be reliably assessed by a passive leg raising (20). Indeed, the passive leg raising increases the mean systemic filling pressure, whereas the resistance to the venous return is unchanged (53). More precisely, in fluid responsive patients, the passive leg raising increases the venous return and in fine the cardiac output by increasing the mean systemic filling pressure to a larger extent than CVP. Conversely, in fluid non-responsive patients, the passive leg raising does not increase the venous return since the mean systemic filling pressure and the CVP increase to a similar extent, resulting in an unchanged pressure gradient for venous return (53). The two most recent meta-analyses pooling respectively 21 studies involving 991 patients (54) and 23 studies involving 1,013 patients (55) found a pooled sensitivity of ≥85% and a pooled specificity >90%, a pooled area under the receiver operating characteristic curve of 0.95 (54,55) and a threshold value of the passive-leg raising-induced increase in cardiac output that predicted fluid responsiveness of 10% (54). Thus, the passive leg raising is now recommended to assess fluid responsiveness in the most recent consensus on circulatory shock and hemodynamic monitoring (56) as well as in the most recent Surviving Sepsis Campaign guidelines (1).
Importantly, the use of passive leg raising to reliably assess fluid responsiveness needs to respect strict rules (57). First, the test should start from the semi-recumbent and not the supine position to sensitize the postural maneuver (58). Second, the effects of the passive leg raising must be assessed on a continuous measurement of cardiac output with a technique that is sensitive enough to track short-term and transient changes of cardiac output. Several invasive or non-invasive techniques of cardiac output measurement can be used for this purpose. It has been shown that the pulse contour derived-cardiac output, as well as the cardiac output measured by esophageal Doppler, transthoracic echocardiography or bioreactance had a similar diagnostic performance (55). Some surrogates of the cardiac output, such as changes in peak velocity of the carotid (59) and femoral (60) arteries, changes in end-tidal carbon dioxide (61-63) and changes in transcutaneous partial pressure of oxygen (64) have also been proposed to reliably assess the effects of the passive leg raising test. It is important to keep in mind that the assessment of the effects of passive leg raising cannot be reliably assessed by the changes in arterial pressure and pulse pressure due to decreased sensitivity (54,55). Finally, some precautions must be taken during the passive leg raising to avoid some confounding factors resulting in adrenergic stimulation, which can induce a misleading interpretation (57).
Conversely to most of the other dynamic tests, the passive leg raising can be used in almost all patients, including those with partial or total spontaneous breathing (54,55,60), atrial fibrillation (65) or low lung compliance (27), as long as the effects of passive leg raising are assessed by a direct measurement of cardiac output. Nevertheless, some limitations and contra-indications must be acknowledged. First, some false negatives have been reported in patients with an intra-abdominal pressure ≥16 mmHg (66,67). Second, scarce data suggested that the use of compression stocking could affect the effects of the passive leg raising (68). Finally, it is obviously contra-indicated to perform a passive leg raising in patients with head trauma and at risk to suffer from intracranial hypertension (69).
Fluid challenge
The fluid challenge technique consists in administering an intravenous bolus of crystalloids or colloids over a short time and in precisely assessing the cardiovascular response to this bolus (70,71). Owing to its inherent infusion of fluids, fluid challenge allows the assessment of fluid responsiveness but not its prediction. Weil and Henning initially proposed to assess the effects of fluid challenge on CVP or PAOP changes over a short time period (70). Nevertheless, as previously discussed, the cardiac filling pressures do not always accurately reflect cardiac preload, precluding their use to reliably assess the effects of the fluid challenge (71). In the same way, the effects of the fluid challenge cannot be reliably assessed by the only fluid-induced changes in arterial pressure (72,73). Indeed, monitoring fluid-induced changes in cardiac output by fluid-induced changes in arterial pressure may result in 22% of false negatives (72). Thus, to prevent any misleading interpretation, the cardiac output should be measured and it is now recommended to measure stroke volume rather than interpreting the fluid-induced changes in CVP or PAOP (1). Non-invasive surrogate of cardiac output such as end-tidal carbon dioxide could also be used (74). In this regard, it has been shown that the maximal effect on cardiac output occurred approximately one minute after the end of the fluid infusion (75).
The FENICE study, a multicenter study conducted among 311 centers across 46 countries around the world, showed a very large variability in the conduction of fluid challenge (12). This variability concerned the indications of the fluid challenge, the volume and the rate of infusion, as well as the markers used to assess the effects of the fluid challenge (12). In brief, arterial hypotension was the main indication for fluid challenge. The median volume of infusion was 500 mL and the median rate of infusion was 24 minutes. Interestingly, the response to fluid challenge was mainly assessed using the fluid-induced arterial pressure changes (12). Despite this variability, the proportion of responders to a fluid challenge was not affected by the type of fluid but decreased when the infusion time was ≥30 minutes (76). It also depended on the amount of fluids administered (77). Finally, no safety parameter was used in 72% of fluid challenges and in half of the cases of a negative fluid challenge, an additional fluid bolus was administered (12). This surprising misinterpretation of the fluid challenge may eventually result in fluid overload.
Alternatively, a mini-fluid challenge consisting in infusing a small volume of fluids has been proposed in the intra-operative room setting (22) as well as in intensive care unit patients (21,78). These studies have shown that either changes in velocity-time integral measured by transthoracic echocardiography (21), pulse contour-analysis derived cardiac output (22), or changes in SVV (78) induced by the infusion of only 100 mL of saline reliably predicted fluid responsiveness. However, because such a small volume of fluid might induce only small hemodynamic changes, a very precise technique is required to measure cardiac output.
How to use the concept of fluid responsiveness at bedside?
The interest of testing fluid responsiveness at bedside is twofold. On the one hand, testing fluid responsiveness may help physicians to know when to start, when to continue but also when to stop fluid administration, especially in patients in whom signs of circulatory failure have disappeared (10). On the other hand, testing fluid responsiveness may help physicians to manage fluid removal (Figure 1).
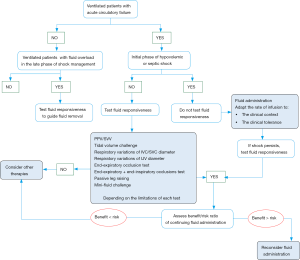
Fluid responsiveness to guide fluid administration
Some important points must be kept in mind. First, it is important to highlight that being preload responsive is a physiological state. In other words, a positive predictive test of fluid responsiveness must not necessarily result in fluid administration. Indeed, as previously discussed, the main goal of fluid administration is to increase the cardiac output and ultimately the oxygen delivery. Thus, the question of fluid administration makes sense only in patients with preload responsiveness and signs of tissue hypoxia (79).
Second, even if it is recommended to predict fluid responsiveness in patients with acute circulatory failure (56), there are some cases where testing fluid responsiveness is not necessary and could even be deleterious by delaying fluid administration. In particular, at the initial phase of hypovolemic or septic shock, hypovolemia is constant and most of the patients should be responsive to initial fluid resuscitation (80). In this regard, we recently proposed a more individualized approach of fluid administration in septic shock patients (3). Within the first hour of resuscitation, fluids should be infused urgently without using any predictor of fluid responsiveness. A rate of around 10 mL/kg within the first hour (e.g., from 30 to 60 minutes) of resuscitation should be reasonable. A higher rate should be considered in cases of evident fluid losses, obvious signs of hypovolemia, or abdominal origin of infection and a lower rate of infusion should be considered if signs of pulmonary edema appear during fluid infusion or in case of severe lung injury (3).
Third, it is now well established that a positive fluid balance is associated with a poor outcome in critically ill patients: the higher the fluid balance, the poorer the outcome (5-9). That is the reason why after the initial fluid resuscitation, when signs of shock persists, the decision of further fluid infusion should be made after individual assessment of its benefits/risks ratio, the dynamic variables of fluid responsiveness being used to predict the expected benefits, in order to prevent harmful effects of fluid overload in fluid unresponsive patients, who represent almost half of the critically ill patients (4). Whether the risks are judged to be greater than the benefits, especially in patients with ARDS, other therapies such as vasopressors infusion should be considered even in the case of preload responsiveness. In such patients, the transpulmonary thermodilution technique could be interesting to guide the fluid management, since it allows the measurement of the extravascular lung water and the pulmonary vascular permeability index (81). Both are independent predictors of mortality and indicate the risk of fluid administration in patients with ARDS (9,81), and could be used as safety parameters.
Fluid responsiveness to guide fluid removal
It is well known that fluid overload may lead to failure of weaning from mechanical ventilation and that fluid removal may decrease the duration of the weaning process (82). Thus, during the late phase of shock management, testing fluid responsiveness may help intensivists for guiding fluid removal in ventilated patients with fluid overload. In this regard, the presence of fluid unresponsiveness assessed by a passive leg raising before starting ultrafiltration during renal replacement therapy could reliably predict a good haemodynamic tolerance of the patients to fluid removal (83).
Conclusions
Since fluid overload has deleterious effects in critically ill patients, prediction of fluid responsiveness is crucial. The static markers of cardiac preload are not reliable predictors of fluid responsiveness. Dynamic tests are better for this purpose than static markers of cardiac preload. Most of the dynamic indices are based on heart-lung interactions and share important limitations. The passive leg raising test is reliable and usable in many situations encountered in the intensive care unit. Nevertheless, it must be kept in mind, that the decision of fluid administration must not only be based only on the presence of fluid responsiveness but also on the presence of signs of tissue hypoxia and on the individual assessment of the benefits/risks ratio of fluid administration.
Acknowledgements
None.
Footnote
Conflicts of Interest: X Monnet and JL Teboul are members of Medical Advisory board of Pulsion/Getinge. M Jozwiak has no conflicts of interest to declare.
References
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Hernández G, Teboul JL. Fourth Surviving Sepsis Campaign's hemodynamic recommendations: a step forward or a return to chaos? Crit Care 2017;21:133. [Crossref] [PubMed]
- Jozwiak M, Hamzaoui O, Monnet X, et al. Fluid resuscitation during early sepsis: a need for individualization. Minerva Anestesiol 2018;84:987-92. [Crossref] [PubMed]
- Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002;121:2000-8. [Crossref] [PubMed]
- Li DK, Wang XT, Liu DW. Association between elevated central venous pressure and outcomes in critically ill patients. Ann Intensive Care 2017;7:83. [Crossref] [PubMed]
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006;34:344-53. [Crossref] [PubMed]
- Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care 2015;19:251. [Crossref] [PubMed]
- Sakr Y, Rubatto Birri PN, Kotfis K, et al. Higher Fluid Balance Increases the Risk of Death From Sepsis: Results From a Large International Audit. Crit Care Med 2017;45:386-94. [Crossref] [PubMed]
- Jozwiak M, Silva S, Persichini R, et al. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med 2013;41:472-80. [Crossref] [PubMed]
- Monnet X, Marik P, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care 2016;6:111. [Crossref] [PubMed]
- Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med 2013;41:1774-81. [Crossref] [PubMed]
- Cecconi M, Hofer C, Teboul JL, et al. Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med 2015;41:1529-37. [Crossref] [PubMed]
- Vieillard-Baron A, Evrard B, Repesse X, et al. Limited value of end-expiratory inferior vena cava diameter to predict fluid responsiveness impact of intra-abdominal pressure. Intensive Care Med 2018;44:197-203. [Crossref] [PubMed]
- Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 2000;162:134-8. [Crossref] [PubMed]
- Feissel M, Michard F, Faller JP, et al. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med 2004;30:1834-7. [Crossref] [PubMed]
- Vieillard-Baron A, Chergui K, Rabiller A, et al. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 2004;30:1734-9. [Crossref] [PubMed]
- Guarracino F, Ferro B, Forfori F, et al. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care 2014;18:647. [Crossref] [PubMed]
- Monnet X, Osman D, Ridel C, et al. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med 2009;37:951-6. [Crossref] [PubMed]
- Jozwiak M, Depret F, Teboul JL, et al. Predicting Fluid Responsiveness in Critically Ill Patients by Using Combined End-Expiratory and End-Inspiratory Occlusions With Echocardiography. Crit Care Med 2017;45:e1131-8. [Crossref] [PubMed]
- Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 2006;34:1402-7. [Crossref] [PubMed]
- Muller L, Toumi M, Bousquet PJ, et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: the mini-fluid challenge study. Anesthesiology 2011;115:541-7. [Crossref] [PubMed]
- Biais M, de Courson H, Lanchon R, et al. Mini-fluid Challenge of 100 ml of Crystalloid Predicts Fluid Responsiveness in the Operating Room. Anesthesiology 2017;127:450-6. [Crossref] [PubMed]
- Michard F, Teboul JL. Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit Care 2000;4:282-9. [Crossref] [PubMed]
- Marik PE, Cavallazzi R, Vasu T, et al. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 2009;37:2642-7. [Crossref] [PubMed]
- Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit Care 2014;18:650. [Crossref] [PubMed]
- Michard F, Chemla D, Teboul JL. Applicability of pulse pressure variation: how many shades of grey? Crit Care 2015;19:144. [Crossref] [PubMed]
- Monnet X, Bleibtreu A, Ferre A, et al. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med 2012;40:152-7. [Crossref] [PubMed]
- Cannesson M, Le Manach Y, Hofer CK, et al. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a "gray zone" approach. Anesthesiology 2011;115:231-41. [Crossref] [PubMed]
- Myatra SN, Prabu NR, Divatia JV, et al. The Changes in Pulse Pressure Variation or Stroke Volume Variation After a "Tidal Volume Challenge" Reliably Predict Fluid Responsiveness During Low Tidal Volume Ventilation. Crit Care Med 2017;45:415-21. [Crossref] [PubMed]
- Myatra SN, Monnet X, Teboul JL. Use of 'tidal volume challenge' to improve the reliability of pulse pressure variation. Crit Care 2017;21:60. [Crossref] [PubMed]
- Min JJ, Gil NS, Lee JH, et al. Predictor of fluid responsiveness in the 'grey zone': augmented pulse pressure variation through a temporary increase in tidal volume. Br J Anaesth 2017;119:50-6. [Crossref] [PubMed]
- Preau S, Dewavrin F, Demaeght V, et al. The use of static and dynamic haemodynamic parameters before volume expansion: A prospective observational study in six French intensive care units. Anaesth Crit Care Pain Med 2016;35:93-102. [Crossref] [PubMed]
- Monnet X, Dres M, Ferre A, et al. Prediction of fluid responsiveness by a continuous non-invasive assessment of arterial pressure in critically ill patients: comparison with four other dynamic indices. Br J Anaesth 2012;109:330-8. [Crossref] [PubMed]
- Feissel M, Michard F, Mangin I, et al. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 2001;119:867-73. [Crossref] [PubMed]
- Monnet X, Rienzo M, Osman D, et al. Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med 2005;31:1195-201. [Crossref] [PubMed]
- Cannesson M, Besnard C, Durand PG, et al. Relation between respiratory variations in pulse oximetry plethysmographic waveform amplitude and arterial pulse pressure in ventilated patients. Crit Care 2005;9:R562-8. [Crossref] [PubMed]
- Sandroni C, Cavallaro F, Marano C, et al. Accuracy of plethysmographic indices as predictors of fluid responsiveness in mechanically ventilated adults: a systematic review and meta-analysis. Intensive Care Med 2012;38:1429-37. [Crossref] [PubMed]
- Barbier C, Loubieres Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med 2004;30:1740-6. [Crossref] [PubMed]
- Machare-Delgado E, Decaro M, Marik PE. Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. J Intensive Care Med 2011;26:116-24. [Crossref] [PubMed]
- Charbonneau H, Riu B, Faron M, et al. Predicting preload responsiveness using simultaneous recordings of inferior and superior vena cavae diameters. Crit Care 2014;18:473. [Crossref] [PubMed]
- Vignon P, Repesse X, Begot E, et al. Comparison of Echocardiographic Indices Used to Predict Fluid Responsiveness in Ventilated Patients. Am J Respir Crit Care Med 2017;195:1022-32. [Crossref] [PubMed]
- Zhang Z, Xu X, Ye S, et al. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol 2014;40:845-53. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Wallace DJ, Allison M, Stone MB. Inferior vena cava percentage collapse during respiration is affected by the sampling location: an ultrasound study in healthy volunteers. Acad Emerg Med 2010;17:96-9. [Crossref] [PubMed]
- Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med 2016;42:1164-7. [Crossref] [PubMed]
- Ma GG, Hao GW, Yang XM, et al. Internal jugular vein variability predicts fluid responsiveness in cardiac surgical patients with mechanical ventilation. Ann Intensive Care 2018;8:6. [Crossref] [PubMed]
- Silva S, Jozwiak M, Teboul JL, et al. End-expiratory occlusion test predicts preload responsiveness independently of positive end-expiratory pressure during acute respiratory distress syndrome. Crit Care Med 2013;41:1692-701. [Crossref] [PubMed]
- Hamzaoui O, Monnet X, Richard C, et al. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit Care Med 2008;36:434-40. [Crossref] [PubMed]
- Georges D, de Courson H, Lanchon R, et al. End-expiratory occlusion maneuver to predict fluid responsiveness in the intensive care unit: an echocardiographic study. Crit Care 2018;22:32. [Crossref] [PubMed]
- Lamia B, Ochagavia A, Monnet X, et al. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med 2007;33:1125-32. [Crossref] [PubMed]
- Maizel J, Airapetian N, Lorne E, et al. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med 2007;33:1133-8. [Crossref] [PubMed]
- Boulain T, Achard JM, Teboul JL, et al. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest 2002;121:1245-52. [Crossref] [PubMed]
- Guerin L, Teboul JL, Persichini R, et al. Effects of passive leg raising and volume expansion on mean systemic pressure and venous return in shock in humans. Crit Care 2015;19:411. [Crossref] [PubMed]
- Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med 2016;42:1935-47. [Crossref] [PubMed]
- Cherpanath TG, Hirsch A, Geerts BF, et al. Predicting Fluid Responsiveness by Passive Leg Raising: A Systematic Review and Meta-Analysis of 23 Clinical Trials. Crit Care Med 2016;44:981-91. [Crossref] [PubMed]
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-815. [Crossref] [PubMed]
- Monnet X, Teboul JL. Passive leg raising: five rules, not a drop of fluid! Crit Care 2015;19:18. [Crossref] [PubMed]
- Jabot J, Teboul JL, Richard C, et al. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med 2009;35:85-90. [Crossref] [PubMed]
- Marik PE, Levitov A, Young A, et al. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest 2013;143:364-70. [Crossref] [PubMed]
- Préau S, Saulnier F, Dewavrin F, et al. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med 2010;38:819-25. [Crossref] [PubMed]
- Monge García MI, Gil Cano A, Gracia Romero M, et al. Non-invasive assessment of fluid responsiveness by changes in partial end-tidal CO2 pressure during a passive leg-raising maneuver. Ann Intensive Care 2012;2:9. [Crossref] [PubMed]
- Monnet X, Bataille A, Magalhaes E, et al. End-tidal carbon dioxide is better than arterial pressure for predicting volume responsiveness by the passive leg raising test. Intensive Care Med 2013;39:93-100. [Crossref] [PubMed]
- Xiao-ting W, Hua Z, Da-wei L, et al. Changes in end-tidal CO2 could predict fluid responsiveness in the passive leg raising test but not in the mini-fluid challenge test: A prospective and observational study. J Crit Care 2015;30:1061-6. [Crossref] [PubMed]
- Xu J, Peng X, Pan C, et al. Fluid responsiveness predicted by transcutaneous partial pressure of oxygen in patients with circulatory failure: a prospective study. Ann Intensive Care 2017;7:56. [Crossref] [PubMed]
- Kim N, Shim JK, Choi HG, et al. Comparison of positive end-expiratory pressure-induced increase in central venous pressure and passive leg raising to predict fluid responsiveness in patients with atrial fibrillation. Br J Anaesth 2016;116:350-6. [Crossref] [PubMed]
- Mahjoub Y, Touzeau J, Airapetian N, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med 2010;38:1824-9. [Crossref] [PubMed]
- Malbrain ML, Reuter DA. Assessing fluid responsiveness with the passive leg raising maneuver in patients with increased intra-abdominal pressure: be aware that not all blood returns! Crit Care Med 2010;38:1912-5. [Crossref] [PubMed]
- Chacko CJ, Wise MP, Frost PJ. Passive leg raising and compression stockings: a note of caution. Crit Care 2015;19:237. [Crossref] [PubMed]
- Monnet X, Teboul JL. Passive leg raising. Intensive Care Med 2008;34:659-63. [Crossref] [PubMed]
- Weil MH, Henning RJ. New concepts in the diagnosis and fluid treatment of circulatory shock. Thirteenth annual Becton, Dickinson and Company Oscar Schwidetsky Memorial Lecture. Anesth Analg 1979;58:124-32. [Crossref] [PubMed]
- Vincent JL, Weil MH. Fluid challenge revisited. Crit Care Med 2006;34:1333-7. [Crossref] [PubMed]
- Monnet X, Letierce A, Hamzaoui O, et al. Arterial pressure allows monitoring the changes in cardiac output induced by volume expansion but not by norepinephrine. Crit Care Med 2011;39:1394-9. [Crossref] [PubMed]
- Pierrakos C, Velissaris D, Scolletta S, et al. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med 2012;38:422-8. [Crossref] [PubMed]
- Lakhal K, Nay MA, Kamel T, et al. Change in end-tidal carbon dioxide outperforms other surrogates for change in cardiac output during fluid challenge. Br J Anaesth 2017;118:355-62. [Crossref] [PubMed]
- Aya HD, Ster IC, Fletcher N, et al. Pharmacodynamic Analysis of a Fluid Challenge. Crit Care Med 2016;44:880-91. [Crossref] [PubMed]
- Toscani L, Aya HD, Antonakaki D, et al. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care 2017;21:207. [Crossref] [PubMed]
- Aya HD, Rhodes A, Chis Ster I, et al. Hemodynamic Effect of Different Doses of Fluids for a Fluid Challenge: A Quasi-Randomized Controlled Study. Crit Care Med 2017;45:e161-8. [Crossref] [PubMed]
- Mallat J, Meddour M, Durville E, et al. Decrease in pulse pressure and stroke volume variations after mini-fluid challenge accurately predicts fluid responsivenessdagger. Br J Anaesth 2015;115:449-56. [Crossref] [PubMed]
- Lira A, Pinsky MR. Choices in fluid type and volume during resuscitation: impact on patient outcomes. Ann Intensive Care 2014;4:38. [Crossref] [PubMed]
- Leisman DE, Doerfler ME, Schneider SM, et al. Predictors, Prevalence, and Outcomes of Early Crystalloid Responsiveness Among Initially Hypotensive Patients With Sepsis and Septic Shock. Crit Care Med 2018;46:189-98. [Crossref] [PubMed]
- Jozwiak M, Teboul JL, Monnet X. Extravascular lung water in critical care: recent advances and clinical applications. Ann Intensive Care 2015;5:38. [Crossref] [PubMed]
- Mekontso Dessap A, Roche-Campo F, Kouatchet A, et al. Natriuretic peptide-driven fluid management during ventilator weaning: a randomized controlled trial. Am J Respir Crit Care Med 2012;186:1256-63. [Crossref] [PubMed]
- Monnet X, Cipriani F, Camous L, et al. The passive leg raising test to guide fluid removal in critically ill patients. Ann Intensive Care 2016;6:46. [Crossref] [PubMed]


