Current advances of targeting HGF/c-Met pathway in gastric cancer
Introduction
In Western world gastric cancer constitutes the fourth most spread cancer type and the second most common cause of cancer death. In 2002 more than 600,000 men and 330,000 women were diagnosed with gastric cancer (1) whereas about 700,000 deaths occurred (2). The prevalence of gastric cancer differentiates geographically with 60% of gastric cancers arising in East Asia (2). Although gastric cancer incidence is decreased in Western countries, the frequency of adenocarcinomas of the gastroesophageal junction (GEJ) has been soaring (2,3). Clinically, the prognosis for advanced gastric cancer is poor and the 5-year overall survival (OS) rates reach about 15% (4,5). Although perioperative treatment might be curative for patients with diagnosed cancer, chemotherapy constitutes the main current therapy for gastric cancer patients (6). Therefore, the elucidation of the molecular background of gastric cancer can provide novel targeted therapies leading to new prognostic and therapeutic avenues.
Gastric cancer is a heterogeneous type of cancer and can be classified into several subgroups based on the anatomical, histological and molecular profile (7). Novel targeted therapies depend on the presence and emerging of molecular targets. For instance, human epidermal growth factor receptor 2 (HER2) and epidermal growth factor receptor (EGFR) are expressed in 30–40% of proximal gastric cancer (8,9) whereas gastroesophageal cancer is characterized by c-Met amplification (10). Distal non-diffuse cancer, which is associated with Helicobacter pylori infection, expresses high levels of vascular endothelial growth factor (VEGF) (11). Molecular aberrations often occur, including fibroblastic growth factor receptor 2 (FGFR2) signaling and phosphoinositide 3-kinase-Akt-mammalian target of rapamycin (PI3K/Akt/mTOR) pathway (12-14). Gastric cancer has recently been divided into five subgroups according to the presence of genomic amplifications; FGFR2 (9.3%), EGFR (7.7%), ERBB2 (7.2%), KRAS (8.8%) and c-Met (4%). All the subgroups with these different molecular alternations constitute the 37% of gastric cancer patients and can be potentially addressed by receptor tyrosine kinase (RTK)/RAS-associated biomolecular treatments (15). Several clinical trials have been conducted administrating monoclonal antibodies, tyrosine kinase inhibitors and mTOR inhibitors to gastric cancer patients. Results so far have revealed that molecular targeting therapy is not as promising as in other cancer types including breast and colorectal cancer. The Trastuzumab for Gastric Cancer (ToGA) was the first international trial for HER2-positive advanced/metastatic gastric or GEJ cancer. ToGA showed that adding trastuzumab plus cisplatin and either capecitabine or fluorouracil improved OS to overall population compared to chemotherapy alone (16). This trial contributed to the establishment of a new standard doublet in HER2-positive patients. Ramucirumab, a fully humanized monoclonal antibody against VEGF receptor 2 is a second-line treatment that is routinely considered for patients with advanced gastro-esophageal cancer providing a favorable toxicity profile. However, the necessity for novel targeted agents needs to be fulfilled.
c-Met pathway is a RTK that after binding its ligand, hepatocyte growth factor (HGF) activates plenty of different molecular signaling pathways. Therefore, it is implicated in the regulation of cellular properties including cell proliferation, invasion and angiogenesis (17). The c-Met pathway is aberrantly activated or overexpressed as it has been observed in tumor biopsies in a variety of malignancies. Deregulation of c-Met is strongly correlated with a poor prognosis and metastatic progression and can usually occur by different mechanisms including gene amplification and increased autocrine or paracrine ligand-mediated stimulation. Recent studies have correlated c-Met overexpression with the progression of carcinomas including lung, ovary, breast, kidney, liver, thyroid, colon and gastric carcinomas (7). More specifically, MET has been proved to be a necessary oncogene as well as a subordinate gene responsible for the metastatic behavior of the malignancies. For all these cancer types c-Met has been reported as an independent prognostic factor for worse outcomes (18-21). All these data support the hypothesis that the HGF/c-Met pathway is a pivotal regulator in cancer and offer an enthralling rational for the deep investigation of targeting c-Met in patients with gastric cancer (7,22).
HGF/c-Met signaling in gastric cancer
The RTK, c-Met is a disulfide heterodimer formed of an extracellular and a transmembrane subunit (23) (Figure 1). HGF, which is the ligand of c-Met with the highest affinity is a pleiotropic cytokine secreted by mesenchymal cells. Once secreted it is inactivated and immediately is activated by a number of proteases to its extracellular heterodimer compartment. Subsequently, HGF binds and activates c-Met on epithelial cells in a paracrine fashion (24). HGF contains high and low-affinity c-Met binding sites. After binding, c-MET is dimerized and is trans-autophosphorylated. c-Met activation initiates molecular interactions resulting in the phosphorylation and activation of various signaling pathways, which affect the cellular properties such as proliferation, apoptosis, motility and invasion. Among these pathways are the PI3K/Akt, Jun amino-terminal kinases (JNKs)/p38 MAPK cascades, signal transducer and activator of transcription 3 (STAT3), nuclear factor-κB (NF-κB) and SRC/FAK (25). The activation of signaling pathways in intermediated through src homology-2 domain (SH2)-mediated interactions [STAT3, the p85 subunit of PI3K, SRC and phospholipase C-γ (PLCγ)] (26-28).
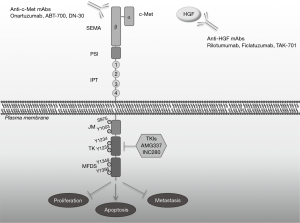
In this process the presence of molecules such as α6β4 integrin, CD44v6 and other RTKs receptors from EGFR family function as modifiers and control the duration and potency of HGF/c-Met signal. In normal conditions, duration and potency depend on the ligand delivery, activation and degradation (29). Accordingly, c-Met undergoes endocytosis and lysosomal proteolytic degradation (30) while retaining its signaling capacity. The inactivation of c-Met is a result of the formation of an N-terminal soluble protein (decoy c-Met) which naturally antagonizes c-Met by insulating HGF leading to an impaired dimerization of the receptor (31-33).
Several preclinical studies have shown that the direct association of c-Met with regulatory signaling pathways promotes cell invasiveness, angiogenesis and metastasis whereas this effect can be reversed after suppression of overexpressed c-Met (34). Additionally, c-Met promotes the proliferation of growth-limited by hypoxia cancers through a negative feedback loop where the hypoxic condition of tissues increases HGF levels (35).
The loss of normal regulation of c-Met results in the oncogenic activation and function of c-Met. It has been reported that in GC, the main cause of inappropriate activation of c-Met pathway is the amplification and mutation of the MET gene with subsequent protein overexpression and kinase activation (24). Other causes for c-Met activation include transcriptional deregulation such as transcriptional upregulation from other oncogenes (K-RAS), inadequate c-Met degradation, ligand-independent activation, autocrine overexpression of HGF ligand or even environmental conditions such as hypoxia and inflammation (35,36). Inappropriate stimulation of c-Met/HGF pathway promotes cellular transformation, epithelial-to-mesenchymal transition (EMT), invasion and metastasis (37,38). So, downregulation and/or inhibition of c-Met significantly diminished the growth, the migration and invasion as well as induced the apoptosis of tumor cells for different in vitro tumor model (39). Additionally, in gastric cancer cells, RNA silencing of c-Met using lentivirus, led to the suppression of peritoneal dissemination demonstrating the proliferative and metastatic role of c-Met in gastric cancer (40). Although genetic mutations of the MET gene have been detected in a subset of patients reaching 1–2% of patients with gastro-oesophageal cancer (41,42), they are exceedingly rare in gastric cancer patients. Preclinical assessments of the mutations (43,44) showed that they are not the common cause of constant c-Met activation. On the contrary, overexpression of c-Met and HGF at both the mRNA and protein level has been demonstrated in several independent studies. For instance, MET amplification has been reported in about 4–10% of gastric tumor patients (45) and accordingly, overexpression of c-Met protein in 50% of advanced gastric cancers (46,47). Immunohistochemistry analysis showed that more than 65% of gastric cancers with increased metastatic potential mainly to the liver, express high levels of c-Met (47,48).
Another well-studied activation of c-Met is the indirect way through its crosstalk with multiple other RTKs. In cancer cellular models, it has been shown that EGFR phosphorylation induces HGF-independent c-Met activation through phosphorylation leading to oncogenic activity (49-51). These data enhance the argument that c-Met is a crucial key target for novel anticancer therapies.
Therapies
The past decade, gastric cancer patients are treated with novel targeted agents of HGF/c-Met pathway (Figure 1). Growing preclinical data provided the molecular knowledge to design inhibitors that have been tested in clinical trials, including anti-HGF and anti-c-Met monoclonal antibodies, whose main goal is to prevent the ligation of HGF to the receptor, receptor dimerization and induce degradation of c-Met. The second category of c-Met inhibitors is small-molecule tyrosine-kinase inhibitors (TKIs), which inhibit the catalytic activity of c-Met and are either selective for the c-Met kinase domain or non-selective kinase inhibitors.
Anti-HGF antibodies
Rilotumumab is a fully humanized IgG2 monoclonal antibody that targets HGF, preventing HGF binding to its receptor and consequently c-Met activation (52). The efficacy and safety of rilotumumab as a single agent or combined with ECX (epirubicin, cisplatin and capecitabine) chemotherapy as first-line treatment has been tested in phase II and III studies in patients with advanced gastric cancer or GEJ cancer. A randomized, double blind phase II study revealed rilotumumab plus ECX exhibited a tolerable safety profile and showed greater activity than placebo plus ECX (53). Based on these results, two phase-III studies (RILOMET-1 and 2) were started to examine the efficacy of the combination of rilotumumab with ECX or cisplatin plus capecitabine in c-Met-positive gastric and oesophagogastric junction cancer patients. The findings of RILOMET-1 showed that inhibition of the c-Met activation with rilotumumab is not effective in providing a better clinical outcome in untreated advanced c-Met-positive gastric cancer patients (54). The primary endpoints of the RILOMET-2 trial were progression free survival (PFS) and OS and the secondary include 12-month survival rate, time to response, time to progression, objective response and disease control rates and safety profile (55). The MEGA phase II study also reveals that additional treatment with panitumumab or rilotumumab increased the toxicity and was less effective compared with mFOLFOX6 (oxaliplatin, folinic acid and fluorouracil) as a monotherapy in patients with HER2-negative advanced gastric cancer or GEJ cancer (56). A randomized phase Ib/II clinical trial is the first study to disclose the beneficial effect of combining rilotumumab with panitumumab in previously treated patients with wild-type KRAS metastatic CRC (57).
Furthermore, ficlatuzumab and TAK-701 are humanized monoclonal antibodies against HGF. They specifically target the soluble HGF, blocking the binding of HGF to c-Met. Phase I/II clinical studies are ongoing for evaluating the tolerability, safety, pharmacodynamics and pharmacokinetics of these anti-HGF antibodies (58,59).
Antibodies against c-Met
Onartuzumab is a humanized monoclonal antibody that binds to the c-Met extracellular domain (60). A double-blind, placebo-controlled, randomized phase II study evaluating the efficacy and safety of a modified FOLFOX6 treatment combined with onartuzumab in patients with metastatic, HER2-negative advanced gastro-esophageal cancer failed to demonstrate a noteworthy improvement in PFS with the addition of onartuzumab (61). Likewise, the randomized phase III MET Gastric study, revealed no significant improvements in median OS duration following the addition of onartuzumab to mFOLFOX6 in patients with HER2-negative and c-Met-positive advanced gastric cancer. In a randomized double-blind phase II study the combination of first-line FOLFOX plus bevacizumab with onartuzumab in patients with metastatic colorectal cancer, did not significantly improve efficacy outcomes in either the intention-to-treat or c-Met positive groups (62).
On the other hand, ABT-700 is a humanized anti-c-Met monoclonal antibody with significant preclinical activity in MET-amplified human xenograft tumors (63). Interestingly, treatment with ABT-700 revealed strong single-agent activity according to findings from a phase I open label study with gastric cancer or GEJ cancer patients, characterized by MET amplification (64). An ongoing phase I study assesses the preliminary efficacy and safety of ABT-700 as monotherapy and in combination with chemotherapy in patients with MET-amplified or c-Met-overexpressing solid tumors (65).
The anti-c-Met monoclonal antibody DN-30 has a high affinity against the c-Met extracellular domain, resulting in promoting ADAM10-dependent proteolytic shedding of the receptor and consequently inducing down-regulation of c-Met (66,67). As a consequence, Ab-induced shedding impairs HGFR signal transduction, inhibits the tumor growth, metastatic potential of gastric cancer cells in vitro and in vivo (66).
Selective c-Met TKIs
AMG-337 is an oral inhibitor that selectively binds to c-Met and inhibits c-Met signaling pathways. A phase Ib/II study about the safety and tolerability of AMG-337 has shown promising results concerning a significant percentage of the subjects, who fulfilled the criteria of having MET-amplified gastric cancer or GEJ cancer (68). An ongoing phase I–II trial study tests the role of AMG-337 when combined with mFOLFOX6 in c-Met-positive advanced-stage patients with stomach or esophageal cancer (69). Furthermore, an ongoing phase Ib clinical trial is currently evaluating the efficacy and safety of INC280, an orally administered c-Met inhibitor, in combination with cetuximab in patients with c-Met-positive metastatic CRC, whose disease progressed on cetuximab or panitumumab treatment (70).
Resistance to inhibitors and the need of stratification biomarkers
Unfortunately, preclinical and clinical data support the development of acquired resistance to HGF/c-Met inhibitors despite an initial response to these therapies. Numerous mechanisms of tumor resistance to anti-HGF and anti-c-Met therapies have been noticed, highlighting the significant challenges on design strategies aiming to overcome or prevent resistance and provide prolonged anticancer effects.
In vitro studies in MET oncogene-addicted gastric cancer cells reveal a major mechanism of resistance to HGF/c-Met targeted therapies, which refers to the increased activation of alternative signaling pathways. This rescue mechanism mainly concerns the application of protein kinase targeted therapy. It has been noticed that the activation of EGFR or HER3 receptor as a result of the increased presence of their ligands, TGFα/EGF or heregulin respectively, was capable of developing resistance to MET inhibition by reactivating PI3K/AKT and/or MEK/MAPK pathways (71-73). Interestingly, half of the MET-amplified EGC patients are characterized by HER2 and/or EGFR co-amplification affecting c-Met inhibitors’ anti-tumor effects (71-73). c-Met and HER2 co-expression enhance tumor aggressive phenotype due to their synergistic effect on cellular invasion (51). Conversely, co-amplification of MET and ligand dependent c-Met activation has been shown to affect the antitumor capacity of lapatinib, a HER2 inhibitor, in HER2-amplified gastro-oesophageal and gastric cancer cells respectively (74,75). These observations arise the need for a combinatorial targeting of EGFR and HGF/c-Met axis to potentially maximize anti-tumorigenic effect for certain MET-oncogene-addicted GC patients (17,76,77).
MET amplification and c-Met activation have also been identified as novel mechanisms of resistance to dual EGFR- BRAF inhibition and cetuximab in BRAF-mutant and KRAS-wild-type colorectal cancer, respectively (78-80). In vitro data highlighted the role of MET and KRAS amplification in resistance to specific c-Met TKIs (81). Another study provided insights into the link between increased c-Met activation and MEK inhibitors treatment in KRAS-mutant and BRAF-mutant CRC, and highlighted the efficient effect of combinatorial targeting of c-Met and MEK on apoptosis in KRAS-mutant models (82,83).
A study based on resistant gastric carcinoma cell revealed a rescue mechanism in c-Met inhibitors that involves the presence of a point mutation (Y1230H) within c-Met activation loop, which constitutes drug target (73). Thus, mutant cells maintain downstream MEK-ERK and PI3K-AKT signaling due to the decreased binding capacity of the inhibitors to the destabilized autoinhibitory conformation of c-Met. Consistently, cells having resistance to c-Met inhibitors are sensitive to MET knockdown. Beyond c-Met’s implication in TKIs resistance, preclinical studies based on CRC establish c-Met as a potential mediator of resistance to radiotherapy and chemotherapy (84,85). Furthermore, patients with gastric cancer exhibited acquired resistance to c-Met inhibition probably due to mesenchymal-to-epithelial transition (86). Data from clinical trials, such as MErCuRIC, which incorporate serial liquid biopsy sampling might shed further light on the mechanisms of secondary resistance that emerge in the clinic.
All together, these findings provide insights into the wide range of resistance mechanisms that cancer cells have the ability to simultaneously develop. There is a great need of widening the molecular and clinical knowledge about these mechanisms in order to find the best therapeutic way to prevent and overcome resistance, identifying those patient populations that are most likely to benefit from treatment with HGF/c-Met targeted therapies.
Conclusions
Despite the research progress on gastric cancer, the fact that the 5-year survival for GC patients remains below 30% reveals the need for novel diagnostic and therapeutic tools. Studies have emerged HGF/c-Met axis as a novel potential target which is related to worse clinical outcomes and thus, can play a catalytic role to move away from ‘one size’ chemotherapy towards personalized cancer therapy. Although ToGA trial has established trastuzumab as a standard therapy in first-line setting HER-2 positive gastric cancer, several target therapies and immunotherapy (Monoclonal antibodies against c-Met, or HGF and selective/unselective c-Met TKIs) are being tested in ongoing clinical trials. However, several major questions remain to be answered such as the definition of the patients’ population that with benefit from such targeted therapies. For instance, although a small cohort of GC patients enrolled in phase II studies for c-Met inhibitors (rilotumumab, onartuzumab) revealed promising data, phase III studies using the same screening criteria for c-Met positivity revealed no survival benefit, regardless the level of c-Met staining. Furthermore, the crosstalk between c-Met and other RTKs, which is responsible for the resistance to c-Met inhibition therapies, suggests that monotherapy is not an effective therapeutic approach but novel combinational strategies should be designed. In summary, although many clinical and preclinical studies support the importance of c-Met as a key-target for GC patients, the immunohistochemical detection of c-Met is not enough to establish c-Met as a reliable biomarker for therapy selection. This reflects the genetic heterogeneity, which underlies gastric cancer and involves several biological factors. The coexistence of genetic alterations within patients’ tumor may affect the response to c-Met targeted agents. Therefore the complemented knowledge with molecular and biochemical studies should not only include new methods to measure c-Met and HGF-dependency but also new tools in order to evaluate the genetic alterations within the tumor. This effort will lead to the identification and selection of the appropriate patient populations that will benefit from the c-Met targeted agents and will provide the directions for the validation of combinational.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [Crossref] [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Bosetti C, Bertuccio P, Levi F, et al. Cancer mortality in the European Union, 1970-2003, with a joinpoint analysis. Ann Oncol 2008;19:631-40. [Crossref] [PubMed]
- Ajani JA. Gastroesophageal cancers: progress and problems. J Natl Compr Canc Netw 2008;6:813-4. [Crossref] [PubMed]
- Goscinski MA, Larsen SG, Warloe T, et al. Adenocarcinomas on the rise--does it influence survival from oesophageal cancer? Scand J Surg 2009;98:214-20. [Crossref] [PubMed]
- Haghighat P, Bekaii-Saab T. An update on biochemotherapy of advanced gastric and gastroesophageal adenocarcinoma. J Natl Compr Canc Netw 2008;6:895-900. [Crossref] [PubMed]
- Schulte N, Ebert MP, Härtel N. Gastric Cancer: New Drugs - New Strategies. Gastrointest Tumors 2014;1:180-94. [Crossref] [PubMed]
- Grabsch H, Sivakumar S, Gray S, et al. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol 2010;32:57-65. [PubMed]
- Lieto E, Ferraraccio F, Orditura M, et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol 2008;15:69-79. [Crossref] [PubMed]
- Janjigian YY, Tang LH, Coit DG, et al. MET expression and amplification in patients with localized gastric cancer. Cancer Epidemiol Biomarkers Prev 2011;20:1021-7. [Crossref] [PubMed]
- Kitadai Y, Sasaki A, Ito M, et al. Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem Biophys Res Commun 2003;311:809-14. [Crossref] [PubMed]
- Hattori Y, Itoh H, Uchino S, et al. Immunohistochemical detection of K-sam protein in stomach cancer. Clin Cancer Res 1996;2:1373-81. [PubMed]
- Kobayashi M, Nagata S, Iwasaki T, et al. Dedifferentiation of adenocarcinomas by activation of phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A 1999;96:4874-9. [Crossref] [PubMed]
- Murayama T, Inokuchi M, Takagi Y, et al. Relation between outcomes and localisation of p-mTOR expression in gastric cancer. Br J Cancer 2009;100:782-8. [Crossref] [PubMed]
- Deng N, Goh LK, Wang H, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 2012;61:673-84. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Appleman LJ. MET signaling pathway: a rational target for cancer therapy. J Clin Oncol 2011;29:4837-8. [Crossref] [PubMed]
- Edakuni G, Sasatomi E, Satoh T, et al. Expression of the hepatocyte growth factor/c-Met pathway is increased at the cancer front in breast carcinoma. Pathol Int 2001;51:172-8. [Crossref] [PubMed]
- Humphrey PA, Zhu X, Zarnegar R, et al. Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am J Pathol 1995;147:386-96. [PubMed]
- Inoue T, Kataoka H, Goto K, et al. Activation of c-Met (hepatocyte growth factor receptor) in human gastric cancer tissue. Cancer Sci 2004;95:803-8. [Crossref] [PubMed]
- Tsuta K, Kozu Y, Mimae T, et al. c-MET/phospho-MET protein expression and MET gene copy number in non-small cell lung carcinomas. J Thorac Oncol 2012;7:331-9. [Crossref] [PubMed]
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374-80. [Crossref] [PubMed]
- Komada M, Hatsuzawa K, Shibamoto S, et al. Proteolytic processing of the hepatocyte growth factor/scatter factor receptor by furin. FEBS Lett 1993;328:25-9. [Crossref] [PubMed]
- Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med 2010;16:37-45. [Crossref] [PubMed]
- Bradley CA, Salto-Tellez M, Laurent-Puig P, et al. Targeting c-MET in gastrointestinal tumours: rationale, opportunities and challenges. Nat Rev Clin Oncol 2017;14:562-76. [Crossref] [PubMed]
- Fixman ED, Fournier TM, Kamikura DM, et al. Pathways downstream of Shc and Grb2 are required for cell transformation by the tpr-Met oncoprotein. J Biol Chem 1996;271:13116-22. [Crossref] [PubMed]
- Pelicci G, Giordano S, Zhen Z, et al. The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene 1995;10:1631-8. [PubMed]
- Zhang YW, Wang LM, Jove R, et al. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 2002;21:217-26. [Crossref] [PubMed]
- Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin Ther Targets 2012;16:553-72. [Crossref] [PubMed]
- Hammond DE, Urbé S, Vande Woude GF, et al. Down-regulation of MET, the receptor for hepatocyte growth factor. Oncogene 2001;20:2761-70. [Crossref] [PubMed]
- Foveau B, Ancot F, Leroy C, et al. Down-regulation of the met receptor tyrosine kinase by presenilin-dependent regulated intramembrane proteolysis. Mol Biol Cell 2009;20:2495-507. [Crossref] [PubMed]
- Michieli P, Mazzone M, Basilico C, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell 2004;6:61-73. [Crossref] [PubMed]
- Nath D, Williamson NJ, Jarvis R, et al. Shedding of c-Met is regulated by crosstalk between a G-protein coupled receptor and the EGF receptor and is mediated by a TIMP-3 sensitive metalloproteinase. J Cell Sci 2001;114:1213-20. Erratum in: J Cell Sci 2014;127:1620. [PubMed]
- Corso S, Migliore C, Ghiso E, et al. Silencing the MET oncogene leads to regression of experimental tumors and metastases. Oncogene 2008;27:684-93. [Crossref] [PubMed]
- Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003;3:347-61. [Crossref] [PubMed]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332-7. [Crossref] [PubMed]
- Liu X, Yao W, Newton RC, et al. Targeting the c-MET signaling pathway for cancer therapy. Expert Opin Investig Drugs 2008;17:997-1011. [Crossref] [PubMed]
- Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol 2011;3:S21-35. [Crossref] [PubMed]
- Avan A, Quint K, Nicolini F, et al. Enhancement of the antiproliferative activity of gemcitabine by modulation of c-Met pathway in pancreatic cancer. Curr Pharm Des 2013;19:940-50. [Crossref] [PubMed]
- Wang XL, Chen XM, Fang JP, et al. Lentivirus-mediated RNA silencing of c-Met markedly suppresses peritoneal dissemination of gastric cancer in vitro and in vivo. Acta Pharmacol Sin 2012;33:513-22. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Dulak AM, Stojanov P, Peng S, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet 2013;45:478-86. [Crossref] [PubMed]
- Graveel C, Su Y, Koeman J, et al. Activating Met mutations produce unique tumor profiles in mice with selective duplication of the mutant allele. Proc Natl Acad Sci U S A 2004;101:17198-203. [Crossref] [PubMed]
- Zeng ZS, Weiser MR, Kuntz E, et al. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett 2008;265:258-69. [Crossref] [PubMed]
- Hara T, Ooi A, Kobayashi M, et al. Amplification of c-myc, K-sam, and c-met in gastric cancers: detection by fluorescence in situ hybridization. Lab Invest 1998;78:1143-53. [PubMed]
- Amemiya H, Kono K, Itakura J, et al. c-Met expression in gastric cancer with liver metastasis. Oncology 2002;63:286-96. [Crossref] [PubMed]
- Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490-9. [Crossref] [PubMed]
- Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481-9. Erratum in: Lancet Oncol 2013;14:e254. [Crossref] [PubMed]
- Fischer OM, Giordano S, Comoglio PM, et al. Reactive oxygen species mediate Met receptor transactivation by G protein-coupled receptors and the epidermal growth factor receptor in human carcinoma cells. J Biol Chem 2004;279:28970-8. [Crossref] [PubMed]
- Jo M, Stolz DB, Esplen JE, et al. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem 2000;275:8806-11. [Crossref] [PubMed]
- Khoury H, Naujokas MA, Zuo D, et al. HGF converts ErbB2/Neu epithelial morphogenesis to cell invasion. Mol Biol Cell 2005;16:550-61. [Crossref] [PubMed]
- Burgess TL, Sun J, Meyer S, et al. Biochemical characterization of AMG 102: a neutralizing, fully human monoclonal antibody to human and nonhuman primate hepatocyte growth factor. Mol Cancer Ther 2010;9:400-9. [Crossref] [PubMed]
- Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol 2014;15:1007-18. [Crossref] [PubMed]
- Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1467-82. [Crossref] [PubMed]
- Doi T, Kang YK, Muro K, et al. A phase 3, multicenter, randomized, double-blind, placebo-controlled study of rilotumumab in combination with cisplatin and capecitabine (CX) as first-line therapy for Asian patients (pts) with advanced MET-positive gastric (G) or GEJ adenocarcinoma: The RILOMET-2 trial. J Clin Oncol 2015;33:abstr TPS226.
- Malka D, Castan F, Francois E, et al. FOLFOX alone or combined to rilotumumab or panitumumab as first-line treatment in patients (pts) with advanced gastroesophageal adenocarcinoma (AGEA): An open-label, randomized phase II trial (PRODIGE 17 ACCORD 20 MEGA). J Clin Oncol 2015;33:abstr 4013.
- Van Cutsem E, Eng C, Nowara E, et al. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin Cancer Res 2014;20:4240-50. [Crossref] [PubMed]
- Jones SF, Cohen RB, Bendell JC, et al. Safety, tolerability, and pharmacokinetics of TAK-701, a humanized anti-hepatocyte growth factor (HGF) monoclonal antibody, in patients with advanced nonhematologic malignancies: First-in-human phase I dose-escalation study. J Clin Oncol 2010;28:abstr 3081.
- Tabernero J, Elez ME, Herranz M, et al. A pharmacodynamic/pharmacokinetic study of ficlatuzumab in patients with advanced solid tumors and liver metastases. Clin Cancer Res 2014;20:2793-804. [Crossref] [PubMed]
- Merchant M, Ma X, Maun HR, et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci U S A 2013;110:E2987-96. [Crossref] [PubMed]
- Shah MA, Cho JY, Huat IT, et al. Randomized phase II study of FOLFOX +/- MET inhibitor, onartuzumab (O), in advanced gastroesophageal adenocarcinoma (GEC). J Clin Oncol 2015;33:abstr 2.
- Bendell JC, Hochster H, Hart LL, et al. A Phase II Randomized Trial (GO27827) of First-Line FOLFOX Plus Bevacizumab with or Without the MET Inhibitor Onartuzumab in Patients with Metastatic Colorectal Cancer. Oncologist 2017;22:264-71. [Crossref] [PubMed]
- Wang J, Goetsch L, Tucker L, et al. Anti-c-Met monoclonal antibody ABT-700 breaks oncogene addiction in tumors with MET amplification. BMC Cancer 2016;16:105. [Crossref] [PubMed]
- Strickler JH, LoRusso P, Yen CJ, et al. Phase 1, open-label, dose-escalation, and expansion study of ABT-700, an anti-C-met antibody, in patients (pts) with advanced solid tumors. J Clin Oncol 2014;32:abstr 2507.
- US National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01472016
- Petrelli A, Circosta P, Granziero L, et al. Ab-induced ectodomain shedding mediates hepatocyte growth factor receptor down-regulation and hampers biological activity. Proc Natl Acad Sci U S A 2006;103:5090-5. [Crossref] [PubMed]
- Schelter F, Kobuch J, Moss ML, et al. A disintegrin and metalloproteinase-10 (ADAM-10) mediates DN30 antibody-induced shedding of the met surface receptor. J Biol Chem 2010;285:26335-40. [Crossref] [PubMed]
- Kwak EL, LoRusso P, Hamid O, et al. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer. J Clin Oncol 2015;33:abstr 1.
- US National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02344810
- US National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02205398
- Bachleitner-Hofmann T, Sun MY, Chen CT, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther 2008;7:3499-508. [Crossref] [PubMed]
- Kwak EL, Ahronian LG, Siravegna G, et al. Molecular Heterogeneity and Receptor Coamplification Drive Resistance to Targeted Therapy in MET-Amplified Esophagogastric Cancer. Cancer Discov 2015;5:1271-81. Erratum in: Cancer Discov 2016;6:1402. [Crossref] [PubMed]
- Qi J, McTigue MA, Rogers A, et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res 2011;71:1081-91. [Crossref] [PubMed]
- Chen CT, Kim H, Liska D, et al. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol Cancer Ther 2012;11:660-9. [Crossref] [PubMed]
- Kim J, Fox C, Peng S, et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Invest 2014;124:5145-58. [Crossref] [PubMed]
- Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008;7:504-16. [Crossref] [PubMed]
- Zucali PA, Ruiz MG, Giovannetti E, et al. Role of cMET expression in non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitors. Ann Oncol 2008;19:1605-12. [Crossref] [PubMed]
- Bardelli A, Corso S, Bertotti A, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov 2013;3:658-73. [Crossref] [PubMed]
- Liska D, Chen CT, Bachleitner-Hofmann T, et al. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin Cancer Res 2011;17:472-82. [Crossref] [PubMed]
- Pietrantonio F, Oddo D, Gloghini A, et al. MET-Driven Resistance to Dual EGFR and BRAF Blockade May Be Overcome by Switching from EGFR to MET Inhibition in BRAF-Mutated Colorectal Cancer. Cancer Discov 2016;6:963-71. [Crossref] [PubMed]
- Cepero V, Sierra JR, Corso S, et al. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res 2010;70:7580-90. [Crossref] [PubMed]
- Carson R, Celtikci B, Fenning C, et al. HDAC Inhibition Overcomes Acute Resistance to MEK Inhibition in BRAF-Mutant Colorectal Cancer by Downregulation of c-FLIPL. Clin Cancer Res 2015;21:3230-40. [Crossref] [PubMed]
- Van Schaeybroeck S, Kalimutho M, Dunne PD, et al. ADAM17-dependent c-MET-STAT3 signaling mediates resistance to MEK inhibitors in KRAS mutant colorectal cancer. Cell Rep 2014;7:1940-55. [Crossref] [PubMed]
- De Bacco F, Luraghi P, Medico E, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst 2011;103:645-61. [Crossref] [PubMed]
- Li Y, Wang J, Gao X, et al. c-Met targeting enhances the effect of irradiation and chemical agents against malignant colon cells harboring a KRAS mutation. PLoS One 2014;9. [Crossref] [PubMed]
- Kim DC, Park KR, Jeong YJ, et al. Resistance to the c-Met inhibitor KRC-108 induces the epithelial transition of gastric cancer cells. Oncol Lett 2016;11:991-7. [Crossref] [PubMed]


