Multi-drug therapy in breast cancer: are there any alternatives?
Targeted drug discovery in cancer research received a paradigm shift in the post-human-genome sequencing era. Identification of novel drug targets by utilizing several ‘-omics’ searches, followed by validation or understanding the mechanism of action of novel drugs by implementing genome editing technology has been considered as one of the major routine practices in the field of drug discovery nowadays. An example of such approach was published recently by Lindeman GJ and co-worker in the Science Translational Medicine (1) where they implemented genomic technologies in understanding the mechanism of action of the drug targeting one of the important pro-survival proteins Mcl-1 in breast cancer (BC).
Among the several types of cancers, BC is the most commonly diagnosed cancer in women. In the last few decades, the mortality rate has been reduced significantly mainly due to the introduction of new therapeutic measures and advanced detection systems. However, till today, BC is considered as the major cause of the cancer related death among the women. According to the recent report of the World Health Organization, more than half of a million women died from BC in 2015 and the rates of incidences are increasing globally in every year. Though the rate of incidences are highest in the developed nation, but the mortality rates are less in comparison to the developing nation indicating further the importance of implementation of the early detection and advanced therapies, which are still not accessible commonly in the developing nations, in curing and/or expanding the life span of the patients.
Based on the gene expression profiling, BC is subdivided into five major subtypes which are, (I) Luminal A, (II) Luminal B, (III) Normal-like, (IV) Triple-negative/basal-like, and (V) HER2-enriched and among these subtypes, the first three are hormone positive which covers around 70% of the global BC cases. These subtypes are less invasive in nature and often treated with neoadjuvant therapy followed by surgery and selective estrogen receptor modulator (SERM) treatment such as Tamoxifen. Such combination therapies have been found to be quite effective in expanding the lifespan of the patients and are often leading to a complete disease free recovery.
Among the remaining two subtypes of BC’s, the HER2 positive breast cancer cells produce higher than physiological level of the human epidermal growth factor receptor 2 (HER2) protein which drives the growth of the cancer cells. In general, HER2 positive BC is more aggressive in comparison to hormone positive subtypes and contributes about 20% of the overall BC cases. Patients with this type of BC treated with a combination therapy which includes chemotherapy, surgery and targeted therapy such a trastuzumab, which is a monoclonal antibody inactivates the HER2 receptors. However, due to the aggressive nature of this type of cancer, the mortality rates are significantly higher if it remains undetected at the early stage of the disease (2).
The triple-negative BC (TNBC), which covers almost 15% of the global BC cases, are difficult to treat and aggressive in nature. The overall survival rate of TNBC patients over the period of 5 years is around 77%, whereas which is around 90% for other types of BC. Recurrences occur mostly within the first 3 years of initial diagnosis and majority of the cases, patients die within 5 years after detection. Attempts in recent years to understand the molecular subtyping indicated that TNBC itself is a heterogeneous disease with a mixture of variety of subtypes marked by their distinct sensitivity to chemotherapy and clinical outcomes (3,4).
A handful of clinical trials is currently going on using drugs targeted to Poly ADP-Ribose polymerase (PARP), Cyclin-dependent kinases, EGFR, Androgen receptor, PI3K/AKT/mTOR, Src and WNT signalling pathways, but, most of the cases the trials are coupled with chemotherapy and/or in combination of drugs, indicating that a single drug is still a longstanding dream for a disease free recovery of TNBC or to control the propagation at the late stage of TNBC (3).
Several attempts were recently made to develop a novel targeted therapy against TNBC and interestingly, most of them were immerged based on the recent analysis of gene expression in TNBC cell lines and tumour samples. Gene expression profile analysis in recent past showed an altered pattern of BRCA1 expression due to promoter methylation along with the mutation of the p53 gene correlated well with the cisplatin resistance tumours. A high expression CD73 was also shown to be related to the resistance to the doxorubicin. Genomic analysis of basal like (BL) subpopulation of primary TNBC tumours indicated mutations in PIK3CA and p53, along with the increased expression of Myc and HIF1-α. Enhanced amplification was also noticed in several other genes such as MCL1, CDK4, JAK2, AKT1 and EGFR provides additional opportunities to develop novel targeted therapies utilizing kinase inhibitors or developing a small molecule inhibitor against a particular protein playing critical roles in regulatory pathways (5-8).
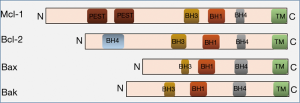
Considering it as a potential target based on previous observations, Lindeman et al. used inhibitors against the anti-apoptotic Bcl family of proteins, Mcl-1. Targeting Mcl-1 in TNBC and Her2 positive breast cancer was considered based on the series of data published previously by several groups. Cumulative evidences indicated that (I) Mcl-l appears to be the principal prosurvival protein in TNBC (9,10), (II) Mcl-1 amplification was frequently observed in TNBC tumours that process the invasive nature after exposed to the neoadjuvant chemotherapy (8), (III) Mcl-1 expression was shown to be associated with the metastasis (11) and (IV) high expression of this protein is coupled with the poor prognosis (12). Downregulation of Mcl-1 in a mouse xenograft model reduced the tumour size coupled with low level of apoptosis (13). Structurally, Mcl-1 is very similar to the other members of the Bcl family of proteins, particularly at the C-terminal part which harbours 3 Bcl-2 homology (BH) domains (Figure 1) confers the ability to expose the hydrophobic groove to form a heterodimer with other members of this family. Mcl-1 exerts its anti-apoptotic effect by binding and sequestering the pro-apoptotic proteins Bcl-2 homologous antagonist killer (Bak) and Bcl-2-associated protein X (Bax) and therefore prevents the release of cytochrome c into the cytoplasm in initiating the proteasome mediated degradation. Intracellular levels of this protein is maintained by the proteasome and non-proteasome mediated degradation during the progression of apoptosis (14).
In order to block the functional activity of Mcl-1, the author used a novel small molecule inhibitor S63845, which binds selectively to the BH3 binding groove of Mcl-1 to impose the functional inactivity and reported to be working both in vitro and in vivo effectively against the Mcl-1 dependent tumours (15). To check the efficiency of this drug, the authors initiated their experiments in monitoring the complex formation of Mcl-1 with its partner proteins such as Bak by ectopic expression of both proteins followed by co-immunoprecipitation. Then they were interested to know the effect of Mcl-1 inactivation on cell lines as well as PDX models in a short time culture assays. Among the cell lines they tested, Her2 positive (SK-BR-3) and TNBC cell lines (MDA-MB-468 and BT-20) were found to be sensitive to this drug whereas, estrogen positive cell lines such as MCF-7 appeared to be insensitive. This observation correlated well with the published data from our laboratory which demonstrated that MCF-7 cells were resistant to induce apoptosis when endogenous Mcl-1 was level was downregulated by siRNA indicating further that apoptotic pathways are diversely regulated in different BC cell lines (16). To validate the effect of this drug on the tumours, the authors utilized their expertise and vast repository of PDX samples which represents almost accurately the architecture and the genomic footprint of the original tumours. A short time survival assay in the presence of S63845 and other BH3 mimetics inhibitors ABT-737 and ABT-199 (inhibit Bcl-2) on Her2 and TNBC PDX collections demonstrated their selective sensitivity only to the S63845.
At this point the authors performed a few experiments to address the mechanism of action of the drug to understand its target as because Mcl-1 is functionally associated in the network of several other proteins such as Noxa, Bid, Bim, Bad and Puma. Her2 positive cell line SK-BR-3 were used to inactivate selectively the BIM, BAD, PUMA independently or in combinations by utilizing CRISPR/cas9 based genome editing method. Single gene inactivation of any of the proteins introduced more sensitivity to S63845 however, deletion in combination such as BIM/BAD and BIM/BAD/PUMA reduced the sensitivity. This experiment is the very first indication that the sensitivity to this drug is not regulated by the single member of this panel of regulatory proteins. Follow-up experiments on S63845 resistant TNBC cell line MDA-MB-468 showed more sensitivity to ABT-199 and ABT-737 treatment. Therefore, an alternative explanation of this phenomenon would be that this drug S63845 is partially affecting the function of the other proteins mentioned above which in turn sensitize the whole regulatory network to the addition of a secondary drugs. A support of this hypothesis emerged form the fact that the S63845 resistant TNBC cell line MDAMB-468 showed enhanced sensitivity to ABT-199 and ABT-737 treatment. An additional support also came from the PDX experiment where two PDX models,one was more sensitive to S63845 than other, were treated with either the drug alone or in combination with ABT-737 or ABT-199 clearly demonstrated the synergistic effect on cell viability. This effect of targeting multiple genes at the same time to induce apoptosis in breast cancer cells was clearly demonstrated in one of our published data where we showed that in MCF-7 cells, apoptosis was aggravated 2–5-fold more when cells were treated with MCL-1 siRNA in combination with ABT-199 (16). In follow-up experiments, both in vitro and using PDX tumour models, Lindeman and his co-worker tried to establish that Mcl-1 inhibition sensitizes further the conventional therapy. Tumours were generated in mouse by implanting two separate TNBC PDX lines, one with BRCA1 mutation (supports more aggressive tumours growth) and a HER2 amplified PDX were treated with either S63845, or cancer chemotherapy drug Docetaxel (inhibits microtubule formation) or Trastuzumab (in case of HER-2 positive PDX) or in combination. Experiments on both models demonstrated that a combination of drugs enhanced the life span of the animals significantly.
Last several decades of cancer research very clearly established that the phenomenon of transformation of somatic cells to cancer cells is associated with a series of changes which mainly include the genetic lesions that lead to enforce uncontrolled signal transduction, loss of regulation of cell cycle checkpoints and the perturbation of the apoptotic pathways that control cell death. On the other hand, however, those changes fuel cancer cells to quickly adapt in an environment if any of the regulatory pathways are blocked by a drug. In majority of the cases, though patients responded well at the beginning but failed to do so in subsequent treatments. For example, in the case of estrogen positive breast cancer cases, more than 25% of the patients, though responded well with SERM at the beginning, generate resistance against the therapy. Analysis of estrogen receptor DNA sequence of patient’s samples identified the accumulation of spontaneous mutations in the ligand binding domain of the estrogen receptor which make them insensitive to the anti-estrogen therapies (17,18). Therefore, the combination therapies are still considered to be the gold standard for the cancer treatment and the data published by Lindeman GL and his co-worker were another strong support to this therapeutic dogma. Several experiments using cell lines, genomic manipulation techniques and at the end PDX-based tumour model proved conclusively that the strongest effect on the BC cells were observed when the Mcl-1 inhibitor was added in the combination with Bcl-2 inhibitors and other anticancer drug Docetaxel. It is important to note that the authors used ABT-737 and ABT-199 which are Bcl-2 inhibitors in combination with S63845 in their PDX experiments where as in the animal experiments Docetaxel was used in combination with S63845 instead of the Bcl-2. It was expected that the combination of ABT-drugs with S63845 would become more suitable combinations because (I) they inhibit Bcl-2 and Mcl-1which play the central role in apoptosis and (II) inactivating both pro-survival proteins at the same time would have been more effective because otherwise inactivation of one could possibly be compensated by the other which authors mentioned as functional redundancy. It was also noted that the combination of drugs generated a significant additive effect in TNBC-PDX-animal model experiment, whereas the effect is quite marginal in Her-2 positive-PDX-animal model, keeping the question alive that how effective this combination therapy would be for Her2 positive BCs. In addition to that, the disease free recovery was also not noticed in either of the cases irrespective of the nature of drug treatments. All those observations, collectively, lead to important questions about rationality of targeting a particular pathway to control the proliferation of cancer cells and evidences so far indicated that it may not be a very efficient.
Now, the question is what would be the fate of cancer drug discovery targeting a particular pathway like this where a greater degree of functional redundancy exists among the participating molecules? More research is needed, perhaps to find out such target molecules which play critical as well as unique roles in several pathways required for the cell survival. Analysis of the systems biology and bioinformatics data should be evaluated more elaborately to identify targets. Special emphasis should be given on the comparison of differentially regulated pathways and altered gene expression pattern to identify novel targets. Such efforts, coupled with appropriate translational model, perhaps would show us a new horizon in targeted drug discovery in cancer.
Acknowledgements
A part of his published data, the author mentioned in the discussion, were generated in the laboratory of Prof. Tom Gonda at the University of Queensland, Brisbane, Australia. Special thanks to Prof. Rik Thompson, Queensland University of Technology, Brisbane, for his encouragement.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Merino D, Whittle JR, Vaillant F, et al. Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci Transl Med 2017.9. [PubMed]
- Loibl S, Gianni L. HER2-positive breast cancer. Lancet 2017;389:2415-29. [Crossref] [PubMed]
- Jhan JR, Andrechek ER. Triple-negative breast cancer and the potential for targeted therapy. Pharmacogenomics 2017;18:1595-609. [Crossref] [PubMed]
- Neophytou C, Boutsikos P, Papageorgis P. Molecular Mechanisms and Emerging Therapeutic Targets of Triple-Negative Breast Cancer Metastasis. Front Oncol 2018;8:31. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61-70. [Crossref] [PubMed]
- Loi S, Pommey S, Haibe-Kains B, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 2013;110:11091-6. [Crossref] [PubMed]
- Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010;28:1145-53. [Crossref] [PubMed]
- Balko JM, Giltnane JM, Wang K, et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov 2014;4:232-45. [Crossref] [PubMed]
- Goodwin CM, Rossanese OW, Olejniczak ET, et al. Myeloid cell leukemia-1 is an important apoptotic survival factor in triple-negative breast cancer. Cell Death Differ 2015;22:2098-106. [Crossref] [PubMed]
- Xiao Y, Nimmer P, Sheppard GS, et al. MCL-1 Is a Key Determinant of Breast Cancer Cell Survival: Validation of MCL-1 Dependency Utilizing a Highly Selective Small Molecule Inhibitor. Mol Cancer Ther 2015;14:1837-47. [Crossref] [PubMed]
- Young AI, Law AM, Castillo L, et al. MCL-1 inhibition provides a new way to suppress breast cancer metastasis and increase sensitivity to dasatinib. Breast Cancer Res 2016;18:125. [Crossref] [PubMed]
- Baglia ML, Cai Q, Zheng Y, et al. Dual specificity phosphatase 4 gene expression in association with triple-negative breast cancer outcome. Breast Cancer Res Treat 2014;148:211-20. [Crossref] [PubMed]
- Campbell KJ, Dhayade S, Ferrari N, et al. MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis 2018;9:19. [Crossref] [PubMed]
- Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett 2010;584:2981-9. [Crossref] [PubMed]
- Kotschy A, Szlavik Z, Murray J, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016;538:477-82. [Crossref] [PubMed]
- Mitra P, Yang RM, Sutton J, et al. CDK9 inhibitors selectively target estrogen receptor-positive breast cancer cells through combined inhibition of MYB and MCL-1 expression. Oncotarget 2016;7:9069-83. [Crossref] [PubMed]
- Li S, Shen D, Shao J, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep 2013;4:1116-30. [Crossref] [PubMed]
- Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 2013;45:1446-51. [Crossref] [PubMed]


