Physiology-guided management of hemodynamics in acute respiratory distress syndrome
Introduction
The diseases and disorders that obligate the need for mechanical ventilation (MV), as well as the conversion from fully spontaneous breathing to positive-pressure ventilation, alter the performance of the heart and circulation. Although these interactions can present problems, skillfully implemented MV may also prove of significant benefit in restoring physiologic homeostasis. Perhaps more than any other intervention employed in critical care, the value and hazards of ventilatory support are determined by the clinician’s mastery of the underlying physiology.
Hemodynamic effects of MV
During both spontaneous and MV, tidal changes in pleural pressure (PPL), transpulmonary pressure (PTP, the difference between alveolar pressure and PPL) and lung volume influence key components of hemodynamics: preload, afterload, heart rate, and myocardial contractility. Changes in PPL affect the preload of the right ventricle (RV) and the afterload of left ventricle (LV), while changes in PTP influence RV afterload and LV preload (1-3). Spontaneous inspiratory efforts produce a negative PPL during inspiration and this reduction in intrathoracic pressure is partially transmitted to the right atrium (RA). In contrast, positive-pressure MV (PPMV) increases intrathoracic pressure and RA pressure during lung inflation—effects that are maintained during the entire respiratory cycle if positive end-expiratory pressure (PEEP) is applied (Figure 1) (2).
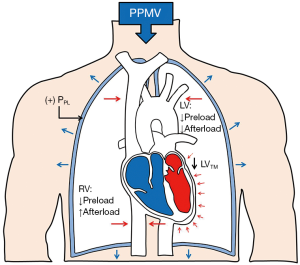
The effect of PPMV on venous return and ventricular function
More than seven decades ago, Cournand et al. (4) studied the effect of changes in PPL on venous return and right heart function. These authors demonstrated that right ventricular (RV) filling related inversely to intrathoracic pressure (or PPL), and as PPL became more positive, venous return and RV preload fell, producing a detectable reduction in cardiac output (4). In terms of variation in stroke volume, the tendency to reduce cardiac output incurred during the inspiratory phase of PPMV is reversed during the expiratory phase; as intrathoracic pressure drops rapidly during expiration, the resulting reduction of PPL and the higher RV net filling pressure allow near complete compensation, provided that expiratory time is of sufficient duration. Expiratory time must equal or exceed inspiratory time in order that the number of heartbeats during expiration may equal or exceed the number that occurs during inspiration (4).
Spontaneous breathing efforts may alter these physiologic dynamics (5). Systemic venous return depends on the pressure gradient between the extrathoracic veins that generate the mean systemic filling pressure (MSFP) and the RA pressure. Spontaneous inspiration raises intrabdominal pressure as it decreases PPL, increasing this MSFP to RA pressure gradient, promoting venous return and thereby boosting RV preload and stroke volume. Conversely, the increase in RA pressure during PPMV impedes venous return; thus, RV preload and hence cardiac output can fall as intrathoracic pressure (or PPL) becomes more positive (1,5).
The findings reported by Cournand and colleagues (4) underscore important features of a “desirable type” of PPMV, which should accomplish: (I) a gradual increase in pressure during inspiration; (II) a rapid drop in pressure after cycling to exhalation occurs; (III) a mean airway pressure (mean PAW) during the expiratory period as near to atmospheric as possible; and (IV) an expiratory time equal to or exceeding the inspiratory time in order to provide adequate ventilation with minimal hemodynamic disturbances. However, in the setting of ARDS, the use of PEEP to improve oxygenation prevents intrathoracic pressure from returning to near atmospheric during expiration. At significant levels, PEEP can reduce cardiac output throughout the respiratory cycle; however, compensatory mechanisms mediated by the sympathetic system often mitigate the negative hemodynamic effects of PPMV by increasing systemic vascular resistance and venous tone, heart rate, and myocardial contractility (4,6).
Some pathologic conditions may potentiate the negative effects of PPMV on cardiac output and venous return, especially when clinically significant levels of PEEP are used. Clinical situations with an absolute or relative reduction in effective blood volume, such as sepsis, distributive shock, hypovolemia, obstructive shock, and dynamic hyperinflation often require volume expansion by administration of intravenous fluids to boost MSFP and augment venous return as well as vasopressors and/or inotropic agents during MV (4-6). PPMV in the setting of complex congenital heart diseases (e.g., single ventricle physiology status post Fontan circulation with an extracardiac conduit) may negatively impact the unique cardiopulmonary shunt physiology and surgical alterations in anatomy that often render pulmonary blood flow a passive process (7,8). PPMV in the setting of complex congenital heart diseases demands a comprehensive understanding of their cardio-pulmonary interactions and a tailored strategy for prompt extubation (7,8).
The concept of transmural pressure (PTM)—defined as the difference between the pressure within a compartment and the pressure that surrounds it, is important to understand how the changes of intrathoracic pressure influence the function of the LV during PPMV (9). For all vascular structures within the thoracic cavity, the PTM is influenced by changes in PPL and respiratory efforts. During spontaneous inspiratory efforts, both PPL and intravascular aortic pressure fall; however, the decrease in PPL is relatively greater than the fall in aortic pressure. As a consequence, PTM effectively increases during systole, resulting in higher LV afterload, potentially with concomitantly lower LV stroke volume if higher preload or increased contractility do not fully compensate (1,4,9). Detrimental effects on afterload, however, are greatly mitigated in healthy individuals by the compensatory augmentation of ejection force (10). Conversely, in patients with ARDS, vigorous inspiratory efforts during PPMV may cause RV crowding of the LV to restrict its diastolic filling while increasing its afterload. Given the increased left ventricular end-diastolic pressure, such changes promote pulmonary edema independently of any effort-related rise in cardiac output (11). Therefore, in severe ARDS with exceedingly high respiratory drive, passive PPMV and PEEP achieved with adequate sedation and/or paralysis (12) could elicit more favorable hemodynamic status by reducing negative inspiratory changes in PPL and by reducing LV afterload (5).
The effect of changes in lung volume on pulmonary vasculature and RV afterload
Transpulmonary pressure (PTP = alveolar minus PPL) is the major determinant of lung volume. On the other hand, pulmonary vascular resistance (PVR) is the main determinant of RV afterload and is directly affected by changes in lung volume. PVR can increase significantly at both extremes of lung inflation—as lung volume increases from residual volume to total lung capacity (TLC), the “alveolar” capillary vessels become increasingly compressed by the lung unit distention, and so their resistance increases. Simultaneously, the resistance of the “extra-alveolar” vessels falls as they become less tortuous and dilate during lung inflation. This combined effect on alveolar and extra-alveolar blood vessels typically generates a “U shaped” relationship between “total PVR” and lung volume (13). During health, these opposing effects of inflation normally optimize at FRC, assuming patency of all lung units (Figure 2) (13). Therefore, the avoidance of large shifts in lung volume relative to FRC during MV is recommended to prevent significant increase in PVR. Unfortunately, this predictability is compromised in ARDS, a condition in which lung collapse and overdistension co-exist to varying degrees throughout the range of TLC.
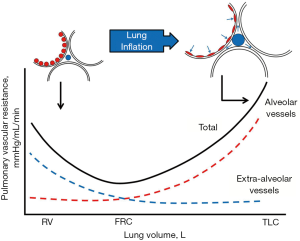
When alveolar pressure exceeds pulmonary venous pressure, microvascular collapse produces West zone 2 conditions (14). Should regional pleural and interstitial pressures exceed pulmonary arterial pressure as well, pulmonary blood flow is largely obstructed through that lung region (West zone 1) (14). Under both conditions, alveolar pressure becomes the outflow pressure for the RV and can considerably increase RV afterload (15). This phenomenon becomes particularly important in septic, post-cardiac surgery, and ARDS patients with decreased RV function and low lung compliance. Under these conditions, the acutely injured lung has reduced capacity to accommodate blood flow, due to inflammation, consolidation, compression of alveolar blood vessels, and microthrombosis (16) while leak across the gas exchanging barrier increases in response to transmural hydrostatic pressure (17). Because a large fraction of total PVR dissipates across the microvasculature (which characteristically has very high permeability), increases of blood flow during ARDS accentuate fluid filtration.
During controlled PPMV, both tidal inflation and PEEP increase PVR and PPL in direct proportion to their effects upon mPAW. Raising mPAW not only increases average lung size but also pushes the chest wall outward by incrementing PPL, thereby impeding venous return. Higher mPAW simultaneously distends lung units already open and encourages microvascular closure of the “alveolar” capillaries. Concomitantly, it recruits additional alveoli. In ARDS patients with highly recruitable lung units, maintaining a relatively open lung while limiting overdistention may improve PVR (13). However, exceedingly high levels of mPAW result in West zone 2 conditions that raise effective vascular resistance within the aerated compartment, redirect blood flow toward poorly ventilated units, increase dead space, and after-load the RV (14). Adequate expiratory time (ideally exceeding the inspiratory time) should be allowed to avoid gas trapping and its adverse hemodynamic effects (4,18).
Ventricular interdependence
Ventricular interdependence implies that changes in intrathoracic pressure and lung volume occurring in one ventricle simultaneously influence the performance of the other. The right and LVs share interwoven myocardial fibers, the interventricular septum and the pericardial space. The interaction between the ventricles is heightened by external compression by the expanded lungs that surround the pericardial fossa. During spontaneous inspiration, increased RV filling causes leftward shift of the interventricular septum and thus impedes diastolic LV filling. Additionally, the increase in RV volume causes the pericardial pressure to rise, and this increment in pressure is then transmitted to the LV, reducing venous return from the pulmonary veins (5,19). These physiologic phenomena are the main causes of exaggerated inspiratory falls in systemic blood pressure (pulsus paradoxus) in cardiac tamponade or pericardial constriction—a process that is accentuated as PPL becomes more negative or with acutely increased RV filling after a fluid bolus; conversely, it is mitigated by the application of PEEP (20,21). Occasionally, elevated right-sided pressures increase shunt through a patent foramen ovale. In severe ARDS, the afterload sensitive RV often overdistends sufficiently to functionally stiffen the interdependent LV (22). If cardiac output remains unchanged, reduced left ventricular compliance produces higher left atrial and pulmonary venous pressures, increasing the tendency for pulmonary edema formation.
Pulmonary vascular mechanics (PVM) in ARDS and ventilator-induced lung injury (VILI)
An optimal PPMV strategy should improve oxygenation and lung compliance by recruiting previously collapsed lung units, while minimizing the negative effects of passive lung inflation on PVR and effective LV compliance—a challenging goal to achieve, especially in the presence of ARDS. VILI is a complex process that is influenced by many factors, and although the great majority of investigative efforts have been directed at elucidating the contribution of airspace mechanics (tidal volume, plateau pressure, and PEEP), adverse effects of PPMV on PVM can influence the development, evolution and severity of VILI (23). For example, experimentally raising pre-capillary vascular pressure intensifies VILI, as does lowering post-capillary pressure if pre-capillary pressure is held constant (24). Large vascular pressure gradients promote the West zone 2 conditions in which microvascular waterfalls (vascular pressure gradients) predispose the vascular endothelium to be injured by poorly tolerated shear stress and/or dissipated energy (14,23).
Experimental observations demonstrate that for a given combination of tidal airway pressures and respiratory rate, reducing vascular flow and flow velocity mitigates capillary stress injury and VILI, independently of their effects on pulmonary vascular pressures (25). Conversely, without changing the magnitudes or profiles of airway and vascular pressures, reducing the frequency of lung stretch improves tissue tolerance (26). Moreover, for a given peak airway pressure and PEEP, hemodynamic support with dopamine may promote lung edema by increasing both cardiac output and pulmonary capillary pressure, as demonstrated by Dreyfuss et al. in an animal model (27). Therefore, limiting peak pulmonary artery pressure (PAP) and flow may diminish lung damage for a given MV strategy (25-27).
In a recent experimental ARDS model, Santos and colleagues tested whether PEEP consistent with an open lung approach (OLA) improves PVM compared to higher or lower PEEP (28). In that study, the PEEP level associated with the highest compliance during a decremental PEEP trial after lung recruitment resulted in best PVM (as measured by effective arterial elastance and vascular compliance) compared to higher or lower PEEP settings (28,29). In concept, the Acute Respiratory Distress Syndrome Network (ARDSnet) approach (30), which aims at limiting VT (adapted to the reduced size of the functional lung) and entails more heterogeneity in the distribution of ventilation due to the persistence of dependent lung collapse, differs from the OLA. The latter aims to increase and maintain the size of the functional lung by alveolar recruitment and individualized adjustment of PEEP (31). The same group of investigators also compared these two commonly applied lung-protective ventilation strategies regarding their effects on PVM and concluded that despite their conceptual differences that define two very distinct lung conditions, the ARDSnet approach and OLA have similar impact on PVM. Higher levels of PEEP used during OLA did not appear to worsen PVM; OLA improved lung mechanics and gas exchange but at the expense of a lower cardiac index explained by the preload-mediated effect of PEEP (6). All of these observations indicate the value of a MV strategy that protects both lung parenchyma and circulation, while reducing ventilator and oxygen demands to avoid adverse two-way interactions between ventilation and perfusion.
Pulmonary microvascular function in RV failure and VILI
Usually in ARDS, pulmonary capillaries are influenced by several dynamic factors including extrinsic obstruction from the interstitial side due to collapsed lung parenchyma (23-25), smooth muscle constriction induced by hypoxia and hypercapnia, eventual vascular remodeling, and the direct effects of applied PPMV on alveolar blood vessels during lung inflation (23). All may directly influence microvascular mechanics. The net result on PVR in ARDS depends on the effective dilatory/constrictory properties of blood vessels within parenchymal regions affected by inflammation and on the state of pulmonary inflation (13,23).
Although we have discussed the effects of both PPMV and changes in lung volume on RV function and the LV, ARDS is indeed a complex condition in which multiple factors hold potential to contribute to the development of RV failure and pulmonary vascular dysfunction during MV. Problems stem from the pathologic processes involving the epithelium of alveoli and blood vessels, as well as vascular injury and obstruction of pulmonary capillaries by micro-thrombi (16). Therefore, the potential for pressure and flow within blood vessels to influence the development and/or evolution of RV failure and VILI also deserves consideration.
As previously shown by Mathieu-Costello and colleagues in laboratory experiments using electron microscopy (32), capillary stress fracture—defined as the mechanical disruption of the microvasculature, occurs when microvascular pressures are elevated to very high levels relative to homeostatic conditions. The pressures necessary to cause capillary stress fracture vary among species (e.g., in rabbits at pressures as low as 40 mmHg) and structural breakdown is more likely to be seen at high lung volumes relative to resting conditions, especially when airway pressure is held constant and the intraluminal vascular pressures upstream and downstream of the alveolus are experimentally made equivalent (33). Regional transmural vascular forces may be dramatically different when mechanically heterogeneous lungs are ventilated with adverse ventilatory patterns, allowing much lower vascular pressures to cause capillary stress fracture, especially lung units and microvessels are degraded by inflammation (34).
Hence, the hemodynamic effects of a given ventilatory strategy are also influenced by the specific condition of the lungs, i.e., stage of ARDS and alveolar collapse or overdistension, in addition to the cardiovascular status of the patient. As ARDS is characterized by altered pulmonary mechanical properties, ventilation/perfusion inhomogeneity, shunt, dead space and HPV, the severity and injurious potential of these hemodynamic effects are hard to predict, and some interventions that attempt to protect the lung may result in both positive and negative effects on pulmonary and systemic hemodynamics.
Current concepts in hemodynamic monitoring in ARDS
Characterization of cardiovascular status and monitoring of hemodynamics are crucial when aiming simultaneously to optimize perfusion, improve gas exchange, and minimize VILI in patients with ARDS and hemodynamic failure. Evaluation of fluid responsiveness is perhaps the first step, and insertion of an arterial catheter allows real-time monitoring of both blood pressure and pulse pressure variation (PPV) (35). When appropriately measured and interpreted, PPV is a good predictor of fluid responsiveness (36,37) by indicating the extent to which stroke volume changes during swings of positive pressure. The predictive value of PPV to indicate fluid responsiveness may be reduced or invalidated by spontaneous breathing activity, low tidal volume, and low lung compliance—all conditions frequently encountered in ARDS. However, if PPV is high (>12%) while tidal volume or lung compliance is low, it is likely that the patient is preload responsive (35-37), meaning that cardiac output should increase with fluid administration and/or decrease with higher levels of PEEP (38). However, the presence of RV failure may result in high values of PPV, indicating RV afterload dependence rather than fluid responsiveness (39). This observation should prompt the clinician to assess RV function with echocardiography and/or to track the changes in PPV during passive leg raising (PLR). A decrease in PPV during PLR suggests fluid responsiveness, whereas no change could indicate the presence of RV afterload dependence (40).
Although central venous pressure (CVP) is a poor predictor of preload responsiveness and is highly influenced by thoraco-abdominal interactions, intra-abdominal pressure and spontaneous breathing (35,41), CVP often helps to monitor the response of RV function to treatment. Measuring the esophageal pressure (PES), a surrogate for PPL, allows estimation of right atrial PTM (right atrial pressure minus PPL), facilitating assessment of how tidal ventilation affects hemodynamics. Unfortunately, routinely measuring PES currently remains a challenge. On the other hand, a central venous catheter (CVC) is required in most cases of ARDS when vasoactive drugs are required and its presence allows measurements of both CVP and central venous oxygen saturation (ScvO2).
Evaluation of PVM and other physiological measurements
Pulmonary artery catheter (PAC)
Placement of PAC allows measurement of PAP and pulmonary artery occlusion pressure (PAOP) and calculation of pulmonary and systemic vascular resistance (42). PAC also allows measurements of mixed venous oxygen saturation (SvO2), estimation of RV cardiac output by thermodilution and RV afterload by PVR calculation. All of these indices serve to assess the response to key interventions such as PEEP titration, volume expansion, and administration of vasoactive drugs. It is worth noting, however, that a high degree of tricuspid regurgitation and RV dilatation are associated with underestimation of cardiac output measured by thermodilution (42,43).
Inserting a PAC to adequately measure PVM becomes well justified in patients with severe ARDS, left ventricular dysfunction or sepsis-associated disorders that do not respond to the initial therapy and require advanced hemodynamic monitoring (42-44). However, mean values of PVR represent only the steady-state opposition to ventricular output, neglecting the pulsatile nature of pulmonary flow and pressure which can significantly contribute to RV load. During PPMV with high PEEP, calculating the transmural value of PAOP (45) allows estimation of true LV filling pressure. Additionally, in the context of ARDS, West zones 1 and 2 can be abnormally extended as a result of a high PTP, especially in cases of hypovolemia. Thus, calculated PVR may underestimate the true resistance of the pulmonary vascular tree and is very dependent on flow (46). In such a context, the vascular pressure gradient across the lungs (mean PAP − PAOP) remains helpful in assessing the degree of pulmonary vascular abnormality (45-47). More specific methods to assess total RV load and pulmonary circulation such as RV strain, pulmonary artery impedance, arterial elastance and advanced instantaneous flow and pressure waveform analysis are needed to more comprehensively assess RV afterload (6,39).
To assess the risk of fluid overload in ARDS, the most useful transpulmonary thermodilution-derived variables are extravascular lung water and pulmonary vascular permeability index (45,48). Additionally, transpulmonary thermodilution serves to calibrate the pulse contour method that allows monitoring cardiac output in real time from a femoral artery pressure curve, but is unable to diagnosed isolated RV failure (49). Multiple invasive (e.g., radial artery catheter) and noninvasive (e.g., volume clamp and applanation tonometry) uncalibrated pulse contour methods have been developed and deployed over the last decade or so; however, their validity has been seriously questioned in the presence of sepsis and/or vasopressor use (35) and such tools cannot be recommended at this time for ARDS patients.
The role of echocardiography
Bedside echocardiography in ARDS allows quick and dynamic assessment of ventricular dimensions and function, changes in cardiac output in response to therapy, variations in vena caval dimensions during the tidal cycle, and preload adequacy by responsiveness to tests such as PLR. Transthoracic echocardiography (TTE) is noninvasive and should be performed early in the course of management of ARDS. However, when TTE is limited by poor echogenicity, transesophageal echocardiography (TEE) may prove helpful. TEE more accurately detects acute cor pulmonale (ACP) than TTE (50). The echocardiographic examination should also include determination of LV ejection fraction, LV end-diastolic area, cardiac output, and markers of LV filling pressures.
During ARDS, comparing the RV end-diastolic area (RVEDA) with the LV end-diastolic area (LVEDA) is useful when assessing RV size (51). An RVEDA/LVEDA ratio between 0.6 and 1 indicates moderate RV dilatation while an RVEDA/LVEDA ratio >1 indicates severe RV dilatation. The combination of a RVEDA/LVEDA ratio >0.6 and the presence of a paradoxical septal motion during end-systole defines ACP (51).
Speckle tracking echocardiography (STE) is a newer and evolving technology also useful in the objective assessment of RV systolic function. STE not only allows more accurate prediction of systolic function but also assessment of the loading conditions (preload and afterload) of the cardiac chambers, specifically of the highly load-dependent RV (52). Additionally, STE is relatively operator independent, with relatively low inter-and intra-observer variability (52,53). When compared with cardiac magnetic resonance (the gold standard for evaluation of RV function), the STE-derived index—RV free wall longitudinal strain (RVFWS)—is considered the best currently available predictor of RV systolic performance (53). It has also demonstrated a close association with invasively measured cardiac index (54) and RV stroke work index (53,54). Recently, Garcia-Montilla and colleagues (55) evaluated whether RVFWS was able to predict an optimal RV filling pressure in patients with ARDS receiving lung protective MV (55). These authors concluded that in terms of RV mechanics, the optimal RV filling pressure in moderate-severe ARDS may typically average CVP of ~13 mmHg, and correlating with a RVFWS of ~20% (normal reference value) (55). Once this technology becomes more widely available and standardized, STE may provide critical care practitioners with an important and objective metric to help with hemodynamic management of patients with ARDS, potentially allowing, for example, real-time PEEP titration based on RV strain.
Hemodynamic management in ARDS
Although left ventricular function is also of interest when managing hemodynamics derangements in mechanically ventilated patient with hypoxemic respiratory failure, the RV is still the cardiovascular structure most directly affected by both PPMV and ARDS. Therefore, therapeutic strategies should be mainly directed at preventing and treating RV dysfunction (Figure 3). In this sense, five general principles should be followed: (I) optimize RV preload; (II) optimize RV systolic function; (III) reduce RV afterload; (IV) maintain an appropriate systemic blood pressure and coronary perfusion; and (V) treat the underlying disease. However, successfully achieving these principles also depends on the mode of MV and the severity of lung dysfunction.
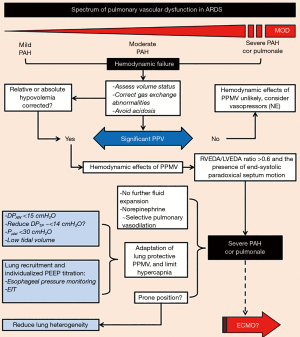
Lung protective MV strategies for prevention of RV failure
Routine RV function assessment facilitates an approach to MV in ARDS patients that is tailored for protection of the RV. This treatment focus, called the ‘RV protective approach’ proposed by Bouferrache and colleagues (56), includes avoidance of alveolar and airway overdistension, maintaining plateau pressure below 27–28 cmH2O, minimizing intrinsic (auto) PEEP, targeting a quasi-normal PaCO2 and ‘low’ PEEP, and routine use of prone positioning (PP) in severe ARDS (56). Some of the physiologic mechanisms by which RV is protected by this strategy are avoiding compression of pulmonary microvasculature by overdistended alveoli (57,58) and reducing the direct increase in PVR resulting from hypercapnia (59). However, unwisely prioritizing low PEEP in an attempt to apply a ‘RV protective approach’ risks increasing RV afterload due to HPV and capillary collapse in the dependent atelectatic regions.
As opposed to the ‘RV protective approach’ proposed by the French group, an OLA aims to promote lung recruitment by using PEEP levels high enough to prevent re-collapse of the newly opened alveoli after a recruitment maneuver. Therefore, OLA decreases pulmonary atelectasis and intrapulmonary shunt but accepts some alveolar overdistension (60,61). Although the ARDSnet approach and OLA have been shown to have a similar impact on PVM (6), a ‘fully recruited’ lung strategy achieved by PEEP titration based on methods targeting pulmonary mechanics alone (disconnected from reasonable oxygenation targets) may result not only in excessively high PEEP levels but also in significant reductions in cardiac index (6). Such an effect may one of the reasons to explain the increased mortality rate reported by recent clinical trials evaluating lung recruitment associated with PEEP titration according to the best respiratory-system compliance (62).
Regardless of the ventilatory strategy applied, two particularly important factors must be considered when assessing and managing hemodynamics in ARDS. First, accurate determination of PTM; direct measurements of intravascular (or intra-ventricular/atrial) pressures must be related to the extravascular pressures. Second, given the pulsatile nature of the circulatory system, in which a non-constant flow is conducted through “elastic tubes”, the simple concept of linear resistance implied by average PVR may be misleading; important phasic changes occur that are produced by tidal inflation—not only on hemodynamics, but also on intravascular and extravascular pressures, circulating blood volume, and proximal and distal pulmonary vascular pressures (6,35,39).
Fluid management in ARDS patients without shock
If effective volume expansion is indicated, isotonic fluids are usually recommended; however, red blood cells could are also effective, and transfusion should be considered in the acutely and critically ill when hemoglobin concentration is less than approximately 8 g/dL (35). Albumin is also an effective alternative to saline, especially in cases of sepsis-related ARDS or severely decreased serum albumin (e.g., liver failure). Since RV failure is the main factor limiting efficacy of fluid administration in restoring perfusion (63), caution is recommended and the risks and the benefits have to be carefully evaluated prior to volume expansion in order to avoid volume overload which may precipitate ACP in the setting of a pre-existing increase of RV afterload (64). In the Fluid and Catheter Treatment Trial (FACTT) of the National Institutes of Health ARDS Network, a conservative fluid protocol (FACTT Conservative) in patients without vasopressor support resulted in a lower cumulative fluid balance and better outcomes than a liberal fluid protocol (FACTT Liberal) (65). The fluid conservative protocol (FACTT Conservative) was associated with a significant increase in ventilator-free days, although it did not demonstrate a reduction in mortality (65). Subsequent ARDS Network studies used a simplified conservative fluid protocol (FACTT Lite) (66). Grissom CK and colleagues (66) compared the performance of FACTT Lite, FACTT Conservative, and FACTT Liberal protocols and concluded that FACTT Lite had a greater cumulative fluid balance than FACTT Conservative but had equivalent clinical and safety outcomes. Therefore, FACTT Lite appears and effective alternative to FACTT Conservative for fluid management in ARDS (65,66).
Management of hemodynamic failure in mechanically ventilated patients with ARDS
Once replete, further fluid expansion in the setting of hemodynamic failure is usually useless and even deleterious. The second step is to look for ACP, which is reported in around 20–25% of ARDS patients (67). Norepinephrine (NE) has been reported to significantly improve RV function by restoring mean arterial pressure and RV blood supply, especially when compromised by high RV wall stress (68,69). Other pharmacologic alternatives to NE include calcium sensitizing agents, such as levosimendan, which have been proposed as therapeutic options to restore the coupling between the RV (inotropic effect) and the pulmonary circulation (vasodilatory effect) (70); However, these agents have the potential to simultaneously promote hypotension. Additionally, two commonly used inhaled selective pulmonary vasodilators—inhaled nitric oxide (5–10 ppm) and inhaled prostacyclin (20–30 ng/kg/min), appear to have comparable efficacy in improving oxygenation, however, there is no evidence for improved clinical outcomes when oxygenation is the main therapeutic goal in unselected populations (71,72). Both agents reduce PVR and improve ventilation/perfusion matching without inducing systemic hypotension. Systemic vasodilators have not been shown to be beneficial in ARDS, and nitric oxide may in fact lead to an increase in renal failure (71-73).
Finally, the beneficial hemodynamics effects of PP deserve special discussion. PP by virtue of its ability to improve the uniformity of ventilation (74) and to attenuate and redistribute the stresses associated with VILI (75), may unload the RV. This could be the main mechanism by which PP improves outcomes in patients with ARDS (76-78). Although the indication for proning has been almost exclusively been considered the severity of ARDS based on the PaO2/FiO2 ratio, it remains to be seen whether a strategy of turning patients prone on the basis of the existence of RV overloading improves outcomes.
Conclusions
Avoiding excessive cardiac demand, regulating fluid balance, optimizing heart rate, and keeping focus on the pulmonary circuit are cornerstones of effective hemodynamic management for patients in all forms of respiratory failure. Functional hemodynamic monitoring that utilizes minimally invasive methods for tracking heart performance and output adequacy is integral to effective care. Attentive management requires a core set of bedside skills, continued vigilance, and mastery of the pathophysiologic principles that inform and guide bedside decisions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marini JJ, Culver BH, Butler J. Mechanical effect of lung distention with positive pressure on cardiac function. Am Rev Respir Dis 1981;124:382-6. [PubMed]
- Wise RA, Robotham JL, Summer WR. Effects of spontaneous ventilation on the circulation. Lung 1981;159:175-86. [Crossref] [PubMed]
- Magder S, Guerard B. Heart-lung interactions and pulmonary buffering: lessons from a computational modeling study. Respir Physiol Neurobiol 2012;182:60-70. [Crossref] [PubMed]
- Cournand A, Motley HL, Werko L, et al. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol 1948;152:162-74. [Crossref] [PubMed]
- Skaburskis M, Helal R, Zidulka A. Hemodynamic effects of external continuous negative pressure ventilation compared with those of continuous positive pressure ventilation in dogs with acute lung injury. Am Rev Respir Dis 1987;136:886-91. [Crossref] [PubMed]
- Santos A, Gomez-Peñalver E, Monge-Garcia MI, et al. Effects on Pulmonary Vascular Mechanics of Two Different Lung-Protective Ventilation Strategies in an Experimental Model of Acute Respiratory Distress Syndrome. Crit Care Med 2017;45:e1157-64. [Crossref] [PubMed]
- Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart 2012;98:1098-104. [Crossref] [PubMed]
- Egbe AC, Connolly HM, Miranda WR, et al. Hemodynamics of Fontan Failure: The Role of Pulmonary Vascular Disease. Circ Heart Fail 2017;10. [Crossref] [PubMed]
- Egbe AC, Connolly HM, Miranda WR, et al. Hemodynamics of Fontan Failure: The Role of Pulmonary Vascular Disease. Circ Heart Fail 2017.10. [PubMed]
- Shepherd JT. The lungs as receptor sites for cardiovascular regulation. Circulation 1981;63:1-10. [Crossref] [PubMed]
- Jardin F, Farcot JC, Boisante L, et al. Influence of positive end-expiratory pressure on left ventricular performance. N Engl J Med 1981;304:387-92. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Whittenberger JL, McGregor M, Berglund E, et al. Influence of the state of the lung on pulmonary vascular resistance. J Appl Physiol 1960;15:878-82. [Crossref] [PubMed]
- Permutt S, Bromberger-Barnea B, Bane HN. Alveolar pressure, pulmonary venous pressure, and the vascular waterfall. Med Thorac 1962;19:239-60. [PubMed]
- Jardin F, Vieillard-Baron A. Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intensive Care Med 2003;29:1426-34. [Crossref] [PubMed]
- Tomashefski JF Jr, Davies P, Boggis C, et al. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol 1983;112:112-26. [PubMed]
- Brigham KL, Woolverton WC, Blake LH, et al. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J Clin Invest 1974;54:792-804. [Crossref] [PubMed]
- Marini JJ. Dynamic hyperinflation and auto-positive end-expiratory pressure: lessons learned over 30 years. Am J Respir Crit Care Med 2011;184:756-62. [Crossref] [PubMed]
- Kelly DT, Spotnitz HM, Beiser GD, et al. Effects of chronic right ventricular volume and pressure loading on left ventricular performance. Circulation 1971;44:403-12. [Crossref] [PubMed]
- Kearns MJ, Walley KR. Tamponade: Hemodynamic and Echocardiographic Diagnosis. Chest 2018;153:1266-75. [Crossref] [PubMed]
- Greenstein YY, Mayo PH. Evaluation of Left Ventricular Diastolic Function by the Intensivist. Chest 2018;153:723-32. [Crossref] [PubMed]
- Frenneaux M, Williams L. Ventricular-arterial and ventricular-ventricular interaction and their relevance to diastolic filling. Prog Cardiovasc Dis 2007;49:252-62. [Crossref] [PubMed]
- Marini JJ, Hotchkiss JR, Broccard AF. Bench-to-bedside review: Microvascular and airspace linkage in ventilator-induced lung injury. Critical Care 2003;7:435-44. [Crossref] [PubMed]
- Broccard AF, Vannay C, Feihl F, et al. Impact of low pulmonary vascular pressure on ventilator-induced lung injury. Crit Care Med 2002;30:2183-90. [Crossref] [PubMed]
- López-Aguilar J, Piacentini E, Villagrá A, et al. Contributions of vascular flow and pulmonary capillary pressure to ventilator-induced lung injury. Crit Care Med 2006;34:1106-12. [Crossref] [PubMed]
- Hotchkiss JR, Blanch LL, Murias G, et al. Effects of decreased respiratory frequency on ventilator induced lung injury. Am J Respir Crit Care Med 2000;161:463-8. [Crossref] [PubMed]
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998;157:294-323. [Crossref] [PubMed]
- Santos A, Lucchetta L, Monge-Garcia MI, et al. The Open Lung Approach Improves Pulmonary Vascular Mechanics in an Experimental Model of Acute Respiratory Distress Syndrome. Crit Care Med 2017;45:e298-305. [Crossref] [PubMed]
- Suarez-Sipmann F, Böhm SH, Tusman G, et al. Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit Care Med 2007;35:214-21. [Crossref] [PubMed]
- Brower RG, Matthay MA, Morris A, et al. Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Kacmarek RM, Villar J, Sulemanji D, et al. Open Lung Approach Network: Open lung approach for the acute respiratory distress syndrome: A pilot, randomized controlled trial. Crit Care Med 2016;44:32-42. [Crossref] [PubMed]
- Mathieu-Costello O, Willford DC, Fu Z, et al. Pulmonary capillaries are more resistant to stress failure in dogs than in rabbits. J Appl Physiol 1995;79:908-17. [Crossref] [PubMed]
- Fu Z, Costello ML, Tsukimoto K, et al. High lung volume increases stress failure in pulmonary capillaries. J Appl Physiol 1992;73:123-33. [Crossref] [PubMed]
- Amato MB, Marini JJ. Barotrauma, volutrauma, and the ventilation of acute lung injury. In: Marini JJ, Slutsky AS. editors. Physiological Basis of Ventilatory Support. New York: Marcel Dekker, 1998:1187-245.
- Vieillard-Baron A, Matthay M, Teboul JL, et al. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 2016;42:739-49. [Crossref] [PubMed]
- Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 2000;162:134-8. [Crossref] [PubMed]
- Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit Care 2014;18:650. [Crossref] [PubMed]
- Michard F, Chemla D, Richard C, et al. Clinical use of respiratory changes in arterial pulse pressure to monitor the hemodynamic effects of PEEP. Am J Respir Crit Care Med 1999;159:935-9. [Crossref] [PubMed]
- Sipmann FS, Santos A, Tusman G. Heart-lung interactions in acute respiratory distress syndrome: pathophysiology, detection and management strategies. Ann Transl Med 2018;6:27. [Crossref] [PubMed]
- Cooke K, Sharvill R, Sondergaard S, et al. Volume responsiveness assessed by passive leg raising and a fluid challenge: a critical review focused on mean systemic filling pressure. Anaesthesia 2018;73:313-22. [Crossref] [PubMed]
- Kim N, Shim JK, Choi HG, et al. Comparison of positive end-expiratory pressure-induced increase in central venous pressure and passive leg raising to predict fluid responsiveness in patients with atrial fibrillation. Br J Anaesth 2016;116:350-6. [Crossref] [PubMed]
- Rajaram SS, Desai NK, Kalra A, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev 2013. [PubMed]
- Balik M, Pachl J, Hendl J. Effect of the degree of tricuspid regurgitation on cardiac output measurements by thermodilution. Intensive Care Med 2002;28:1117-21. [Crossref] [PubMed]
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-815. [Crossref] [PubMed]
- Teboul JL, Pinsky MR, Mercat A, et al. Estimating cardiac filling pressure in mechanically ventilated patients with hyperinflation. Crit Care Med 2000;28:3631-6. [Crossref] [PubMed]
- Zapol WM, Jones R. Vascular components of ARDS. Clinical pulmonary hemodynamics and morphology. Am Rev Respir Dis 1987;136:471-4. [Crossref] [PubMed]
- Bull TM, Clark B, McFann K, et al. National Institutes of Health/National Heart, Lung, and Blood Institute ARDS Network. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med 2010;182:1123-8. [Crossref] [PubMed]
- Jozwiak M, Silva S, Persichini R, et al. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med 2013;41:472-80. [Crossref] [PubMed]
- Monnet X, Teboul JL. Transpulmonary thermodilution: advantages and limits. Crit Care 2017;21:147. [Crossref] [PubMed]
- Lhéritier G, Legras A, Caille A, et al. Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: a multicenter study. Intensive Care Med 2013;39:1734-42. [Crossref] [PubMed]
- Vieillard-Baron A, Prin S, Chergui K, et al. Echo-Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med 2002;166:1310-9. [Crossref] [PubMed]
- Potter E, Marwick TH. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc Imaging 2018;11:260-74. [Crossref] [PubMed]
- Salvo GD, Pergola V, Fadel B, et al. Strain Echocardiography and Myocardial Mechanics: From Basics to Clinical Applications. J Cardiovasc Echogr 2015;25:1-8. [Crossref] [PubMed]
- Kossaify A. Echocardiographic Assessment of the Right Ventricle, from the Conventional Approach to Speckle Tracking and Three-Dimensional Imaging, and Insights into the “Right Way” to Explore the Forgotten Chamber. Clin Med Insights Cardiol 2015;9:65-75. [Crossref] [PubMed]
- Garcia-Montilla R, Imam F, Miao M, et al. Optimal right heart filling pressure in acute respiratory distress syndrome determined by strain echocardiography. Echocardiography 2017;34:851-61. [Crossref] [PubMed]
- Bouferrache K, Vieillard-Baron A. Acute respiratory distress syndrome, mechanical ventilation, and right ventricular function. Curr Opin Crit Care 2011;17:30-5. [Crossref] [PubMed]
- Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med 2007;33:444-7. [Crossref] [PubMed]
- Vieillard-Baron A, Loubieres Y, Schmitt JM, et al. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol 1999;87:1644-50. [Crossref] [PubMed]
- Viitanen A, Salmenpera M, Heinonen J. Right ventricular response to hypercarbia after cardiac surgery. Anesthesiology 1990;73:393-400. [Crossref] [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Beneficial effects of the "open lung approach" with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med 1995;152:1835-46. [Crossref] [PubMed]
- Borges JB, Okamoto VN, Matos GF, et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome . Am J Respir Crit Care Med 2006;174:268-78. [Crossref] [PubMed]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]
- Schneider AJ, Teule GJ, Groeneveld AB, et al. Biventricular performance during volume loading in patients with early septic shock, with emphasis on the right ventricle: a combined hemodynamic and radionuclide study. Am Heart J 1988;116:103-12. [Crossref] [PubMed]
- Pinsky MR. My paper 20 years later: effect of positive end-expiratory pressure on right ventricular function in humans. Intensive Care Med 2014;40:935-41. [Crossref] [PubMed]
- Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564-75. [Crossref] [PubMed]
- Grissom CK, Hirshberg EL, Dickerson JB, et al. Fluid management with a simplified conservative protocol for the acute respiratory distress syndrome. Crit Care Med 2015;43:288-95. [Crossref] [PubMed]
- Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 2016;42:862-70. [Crossref] [PubMed]
- Guyton AC, Lindsey AW, Gilluly JJ. The limits of right ventricular compensation following acute increase in pulmonary circulation resistance. Circ Res 1954;2:326-32. [Crossref] [PubMed]
- Vlahakes GJ, Turley K, Hoffman JI. The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation 1981;63:87-95. [Crossref] [PubMed]
- Morelli A, Teboul JL, Maggiore SM, et al. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med 2006;34:2287-93. [Crossref] [PubMed]
- Dzierba AL, Abel EE, Buckley MS, et al. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or the acute respiratory distress syndrome. Pharmacotherapy 2014;34:279-90. [Crossref] [PubMed]
- Afshari A, Bastholm Bille A, Allingstrup M. Aerosolized prostacyclins for acute respiratory distress syndrome (ARDS). Cochrane Database Syst Rev 2017;7. [PubMed]
- Gebistorf F, Karam O, Wetterslev J, et al. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev 2016;6. [PubMed]
- Vieillard-Baron A, Rabiller A, Chergui K, et al. Prone position improves mechanics and alveolar ventilation in acute respiratory distress syndrome. Intensive Care Med 2005;31:220-6. [Crossref] [PubMed]
- Broccard A, Shapiro RS, Schmitz LL, et al. Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Crit Care Med 2000;28:295-303. [Crossref] [PubMed]
- Vieillard-Baron A, Charron C, Caille V, et al. Prone position unloads the right ventricle in severe ARDS. Chest 2007;132:1440-6. [Crossref] [PubMed]
- Jozwiak M, Teboul JL, Anguel N, et al. Beneficial hemodynamic effects of prone positioning in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2013;188:1428-33. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]


