DPP-4 inhibitors: a promising therapeutic approach against Alzheimer’s disease
Introduction
Alzheimer’s disease (AD), the most common neurodegenerative disorder, presents the main cause of dementia in aging adults. The key pathological features of AD include the accumulation of extracellular plaques composed of amyloid-β (Aβ) and intracellular neurofibrillary tangles containing hyper-phosphorylated tau protein. Clinically, AD usually starts with subtle short-term memory deficits and gradually progresses to a more generalized cognitive impairment, including language disturbance, disorientation in space and time, as well neuropsychiatric symptoms, such as agitation, apathy and delusions (1). Finally, patients may become incapable of performing daily living activities, resulting in a major emotional and financial burden imposed on them and on their caregivers (2).
Unfortunately, currently approved medications including the acetylcholinesterase inhibitors (AChEI) rivastigmine, donepezil and galantamine, as well as the N-methyl D-aspartate (NMDA) receptor antagonist memantine, provide mainly symptomatic benefits and fail to counteract the disease progression. Moreover, the important adverse central and peripheral cholinergic effects observed with AChEI limit their clinical use (3). Notably, it is estimated that the prevalence of the disease and the subsequent care costs for AD patients will double by mid-century (4), highlighting the urgent need for the development of effective and well-tolerated novel treatment.
Despite years of extensive research efforts, the AD pathogenesis still remains elusive. Rare autosomal dominant mutations have been identified in Amyloid protein precursor (APP), presenilin-1 (PSEN1), and presenilin-2 (PSEN2) genes, typically causing early-onset (<65 years) AD (5). However, the majority of AD cases are sporadic with unknown etiology, although several risk factors contribute to its development (6).
Interestingly, accumulating epidemiological evidence has revealed a clear link between cardiovascular risk factors and AD. In particular, type 2 diabetes mellitus (T2DM) patients as well as pre-diabetics seem to have a two-fold higher risk of developing AD, independently of other vascular risk factors (6,7). Pathophysiologically, AD and T2DM share several common characteristics, including insulin resistance in the periphery and the brain, amyloid aggregation and degeneration in brain and pancreas, microvascular damage, oxidative stress, mitochondrial dysfunction, production of advanced glycation end products (AGEs) and excessive inflammatory response (6,8,9). At a molecular level, insulin signaling impairment, abnormally higher activity of glycogen synthase kinase-3 (GSK-3) and the subsequent deregulated protein phosphorylation have been detected both in AD and T2DM patients. Therefore, it has been suggested that pharmacological agents against T2DM could also be beneficial for the prevention and/or treatment of AD (6,9).
Indeed, recent clinical studies indicate that intranasal insulin administration may exert beneficial effects on cognitive performance of AD patients. Moreover, metformin, another anti-diabetic drug, has been associated with a lower risk of developing cognitive impairment (10). Likewise, animal studies have shown that glucagon-like peptide-1 (GLP-1) analogs, such as exendin-4 and liraglutide, an established treatment option for T2DM, may attenuate memory deficits and diminish β amyloid plaque load in the brain, thus protecting against neuronal cell death (11).
GLP-1 is an incretin 30-amino acid peptide originating from preproglucagon that is secreted by neuroendocrine L-cells in the gut after food consumption and by neuronal cells in the brain (12,13). It acts through GLP-1 receptors (GLP-1R) that belong to the G-protein-coupled receptor (GPCR) superfamily, found in many peripheral tissues as well as in central nervous system (CNS) (14). Upon GLP-GLP-1R interaction, activation of adenylyl cyclase takes place, stimulating cAMP production and protein kinase A activation, thus affecting several cellular functions (14,15). GLP-1 exerts its anti-diabetic effects via stimulation of glucose-dependent insulin release, suppression of glucagon secretion and inhibition of pancreatic beta-cell apoptosis (12,13). However, endogenous GLP-1 is inactivated rapidly by DPP-4, leading to a very short circulating half-life of less than 2 minutes (16). Consequently, GLP-1 analogs, such as liraglutide and exenatide that are resistant to protein cleavage, as well as DPP-4 inhibitors, such as saxagliptin, linagliptin and vildagliptin which extend plasma half-life of GLP-1, induce a functional enhancement of GLP-1. DPP-4, being a proteolytic enzyme that is expressed in most cell types, exists in both membrane-anchored and soluble form (6,17). DPP-4 is found in blood plasma and cerebrospinal fluid and exerts its multifunctional effects through various signaling pathways that induce and regulate inflammatory and immunological processes (6). It has the capacity to degradate GLP-1 as well as several other peptides, including glucose-dependent insulinotropic polypeptide (GIP), brain natriuretic peptide (BNP), substance P, neuropeptide Y, and stromal derived factor-1α (SDF-1α) (18).
Notably, DPP-4 inhibitors have been demonstrated recently to display important neuroprotective effects, reverse pathophysiologic processes observed in AD, and improve cognitive performance of AD animal models and patients. This narrative review discusses recent evidence on the molecular and functional effects of DPP-4 inhibitors on AD development and their potential therapeutic efficacy.
In vitro evidence of DPP-4 inhibitors effects in AD
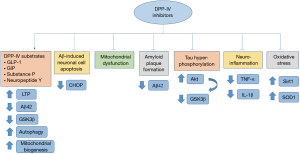
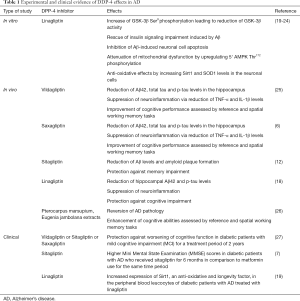
Recent studies demonstrated that DPP-4 inhibitors can significantly suppress the neurodegenerative processes observed in AD via multiple molecular mechanisms in vitro (Table 1) (19-21). Specifically, linagliptin was shown to rescue insulin signaling impairment induced by Aβ, leading to a reduction of tau hyper-phosphorylation (19). More specifically, linagliptin restored Tyr phosphorylation levels of insulin receptor substrate-1 (IRS-1) leading to higher levels of v-Akt Murine Thymoma Viral Oncogene/Protein kinase-B (Akt/PKB) and a subsequent reduction of GSK-3β activity (19). GSK-3β is a proline-directed serine/threonine kinase with well-established implication in AD pathogenesis, mainly via hyper-phosphorylation of tau protein (20), elevation of Aβ production, neuroinflammation, reduction of acetylcholine synthesis (20) and induction of oxidative stress (21).
Full table
Additionally, linagliptin has been shown to effectively inhibit Aβ-induced neuronal cell apoptosis (19). The underlying molecular mechanism is still unclear. However, DPP-4 inhibitors are known to suppress pancreatic beta cell apoptosis, via downregulation of Tribbles Pseudokinase 3 (TRIB3), activating transcription factor 4 (ATF-4) and C/EBP homologous protein (CHOP), three components of the endoplasmic reticulum (ER) stress-mediated apoptosis pathway (22). Interestingly, ER stress and CHOP upregulation are main contributors of AD pathogenesis, since activated CHOP may increase Aβ levels, induce reactive oxygen species (ROS) accumulation and promote neuroinflammation (23). Therefore, it is suggested that the anti-apoptotic effects of DPP-4 inhibitors observed in AD are mediated through ER stress-induced signaling pathways.
Moreover, as DPP-4 inhibitors increase GLP-1 levels, it is possible that DPP-4 neuroprotective properties against Aβ toxicity could be at least partially mediated through the effects of GLP-1-GLP-1R interaction within the CNS. Indeed, GLP-1 was shown to act as a neurotrophic factor and protect against neurodegeneration, possibly by promoting long-term potentiation (LTP), enhancing neurite outgrowth and contributing to synapse formation, in a manner that resembles nerve growth factor (NGF) (14). Given the fact that synaptic loss is a key pathophysiological feature of AD (14), DPP-4 inhibitors via GLP-1 elevation may hinder AD progression. Furthermore, GLP-1 seems to reduce Aβ-induced hippocampal neuronal cell death in vitro (28). Accordingly, another study revealed that GLP-1 is able to reduce the levels of Aβ, as well as the concentration of secreted and intracellular APP, the amyloid precursor protein which upon proteolysis induces Aβ formation (29). Furthermore, it has been demonstrated that GLP-1 analogues may reverse tau hyper-phosphorylation induced by AGEs mainly via down-regulation of GSK-3β (21). AGEs are known to be highly implicated in AD pathogenesis through several mechanisms, including acceleration of Aβ deposition, APP expression, abnormal tau phosphorylation and oxidative stress (21). Therefore, since abnormal Aβ accumulation and tau hyperphosphorylation are considered to play a key role in AD progression, DPP-4 inhibitors via GLP-1 elevation may prove beneficial towards AD treatment.
Furthermore, linagliptin was shown to attenuate mitochondrial dysfunction and demonstrate anti-oxidative effects in vitro by upregulating 5’ AMP-activated protein kinase (AMPK) and contributing to increased sirtuin 1 (Sirt1) and superoxide dismutase 1 (SOD1) levels in neuronal cells (19). These data agree with the findings of another study reporting that linagliptin and to a lesser extent other DPP-4 inhibitors exert anti-oxidative properties via the suppression of ROS formation and of NADPH oxidase subunits expression in vascular and cardiac tissues of rats (24). Accordingly, it has been documented that GLP-1 analogues may enhance mitochondrial biogenesis and oxidative stress, mainly by increasing mitochondrial membrane potential and activating peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) signaling pathway in both AD in vitro and in vivo models (21). Since mitochondrial damage and oxidative stress form a vicious cycle in AD pathophysiology (21), it is proposed that targeting this process through DPP-4 inhibitors could be a potentially effective therapeutic strategy against AD progression.
On the contrary, it has been demonstrated that DPP-4 is able to cleave the synthetic amyloid peptides Aβ40 and Aβ42 in vitro, inhibit their aggregation and disaggregate the preformed amyloid fibrils (30). Based on this finding, we could postulate that DPP-4 inhibitors could decrease the ability of DPP-4 to cleave Aβ and in turn exert detrimental effects on AD pathogenesis. These contradictory data may be explained by the findings of Piccini et al. who demonstrated that the molecular composition of the N-terminal domain of Aβ in AD patients differs from that of normal elder individuals, contributing to its neurotoxicity (31). Moreover, it is also well-known that monomeric Aβ40 and Aβ42 are required for the synaptic plasticity and neuronal survival in normal brain, inducing memory formation (32). Therefore, it would be worth investigating the differential effects of DPP-4 inhibitors in Aβ deposition in AD patients, compared to healthy younger and aged controls in order to clarify the above-mentioned data.
In vivo evidence of DPP-4 inhibitors effects in AD
The neuroprotective role of DPP-4 inhibitors is further supported by recent in vivo evidence revealing their ability to reverse cognitive impairment and restore the cardinal neurodegenerative processes in AD animal models (Table 1). In particular, it has been shown that vildagliptin or saxagliptin treatment decreased Aβ42, total tau and p-tau levels in the hippocampus of rat models of AD. Furthermore, these DPP-4 inhibitors were shown to suppress neuroinflammation, and these alterations were also accompanied by improvement of the cognitive performance of the animals (6,25,26). In accordance, sitagliptin exerted similar effects by reducing Aβ levels and amyloid plaque formation, as well as by protecting against memory impairment in a murine AD model (12). In the same context, another study revealed that linagliptin reduced hippocampal Aβ42 and p-tau levels, hindered neuroinflammation and inhibited cognitive decline in a mouse AD model (18). Interestingly, it has been reported that the use of Pterocarpus marsupium and Eugenia jambolana extracts, two medicinal plants with DPP-4 inhibitory features, enhanced cognitive abilities and reversed AD pathology in a rat AD model (26). Consequently, it is also possible that herbal-based therapies with DPP-4 inhibitory properties may prove effective against AD.
Since linagliptin is not able to cross blood brain barrier (BBB) easily, it is possible that its neuroprotective effects could be exerted at least partially by increasing the bioactivity of GLP-1, which can pass through BBB effectively (18,19). Indeed, saxagliptin, vildagliptin, linagliptin and sitagliptin treatment was found to increase GLP-1 expression in the hippocampus of animal AD models (6,18,25,26). As previously described, GLP-1 can interact with GLP-1R found in the hippocampus, cerebral cortex, brainstem, cerebellum and other brain regions (6,13). GLP-1R agonists were found to reduce amyloid burden, tau levels and activated microglia in the brain of animal models of AD, as well as to inhibit memory decline. Therefore, this is additional in vivo evidence supporting the efficacy of DPP-4 inhibitors in reversing AD pathology via GLP-1 elevation (33-35).
However, a part of GLP-1, DPP-4 could also exert beneficial neuroprotective effects via elevation of GIP, a peptide that is produced in gastrointestinal K cells, acts synergistically with GLP-1 and is further proteolyzed by DPP-4 (14,36). Interestingly, GIP analogues were found to inhibit cognitive dysfunction, protect hippocampal synaptic plasticity, reduce amyloid plaque burden and suppress neuroinflammation in animal AD models (36). Furthermore, SDF-1α, another DPP-4 substrate, has been demonstrated to chemo-attract microglia from bone marrow into the brain of a mouse AD model, leading to a reduction of Aβ accumulation, via enhancement of Aβ phagocytosis (37). Additionally, substance P that is also cleaved by DPP-4 seems to display neuroprotective roles against AD, since it is able to promote non-amyloidogenic processing of APP and inhibit Aβ deposition perhaps by regulating voltage-gated potassium (Kv) channel currents (38). Finally, neuropeptide Y was found to enhance autophagy, reduce excitotoxicity and attenuate neuroinflammation, with a well-established role in AD pathogenesis (39). Consequently, DPP-4 inhibitors are shown to exert pleiotropic neuroprotective effects in AD, and further studies are warranted in order to elucidate the underlying molecular mechanisms.
Clinical evidence of DPP-4 inhibitors effects in AD
Apart of the experimental in vitro and in vivo evidence discussed above, the beneficial effects of DPP-4 inhibitors have also been shown in some clinical studies of AD (Table 1) (7). First of all, a retrospective longitudinal study reported that a two years use of DPP-4 inhibitors was significantly associated with a protection against cognitive function deterioration in diabetic patients with mild cognitive impairment (MCI) (27). Although this study included patients with MCI and not AD, it gave us valuable evidence regarding the possible neuroprotective function of DPP-4 inhibitors in AD patients. Indeed, some years later, a perspective observational study reported that diabetic patients with AD that received sitagliptin displayed significantly higher Mini Mental State Examination (MMSE) scores in comparison to diabetic patients with AD who also received metformin during treatment period (7).
Furthermore, it was shown that after linagliptin treatment, diabetic patients suffering from AD displayed increased expression of Sirt1, a known anti-oxidative and longevity factor, in their peripheral blood leucocytes (19). Sirt1, a class III histone deacetylase (HDAC) exhibits neuroprotective properties by promoting synaptic plasticity and enhancing memory through regulation of cAMP response elements (CREB) and Brain-derived neurotrophic factor (BDNF) expression (40). Likewise, Sirt1 has been suggested to reduce Aβ accumulation, suppress oxidative stress and inhibit neuronal loss in vitro and in vivo (41). Interestingly, serum Sirt1 concentration was found significantly reduced in patients suffering from AD in comparison to controls (42). Thus, we can speculate that serum Sirt-1 levels could also be used as biomarkers of the efficacy of DPP-4 inhibitors in AD patients’ treatment.
Concluding remarks
It is clear that AD is a multifactorial and yet incurable disease, demanding a treatment approach that targets simultaneously multiple molecular mechanisms with minimal side effects. Current treatment strategies against AD aim mainly at (I) reducing Aβ formation and inhibiting Aβ aggregation via various mechanisms, such as β- and γ-secretase inhibition or anti-amyloid immunotherapy (amyloidocentric approach) and (II) anti-tau immunotherapy and hindering tau hyper-phosphorylation (43). However, despite the effectiveness of many of these strategies in animal models, clinical trials have failed to demonstrate their efficacy till now while some of these medications exhibit serious adverse reactions, including meningoencephalitis after Aβ immunotherapy (43,44).
Based on the above data, it is evident that DPP-4 inhibitors may affect core pathogenic processes in AD, including Aβ accumulation; Aβ induced neuronal cell apoptosis, tau hyperphosphorylation, mitochondrial dysfunction, oxidative stress and neuroinflammation while they can potentially inhibit AD progression and cognitive decline (Figure 1). Since DPP-4 inhibitors are considered safe for long-term use and are generally well-tolerated, they could be a promising therapeutic approach against AD (6). In particular, they display a lower hypoglycemia risk in comparison to other anti-diabetic medications and have been correlated with no significant alterations in body weight, as well as a reduced need for insulin (7). On the other hand, GLP-1 analogues were found to decrease appetite, which is beneficial for obese T2DM patients, but possibly not in AD patients (36). Given the common comorbidity with T2DM, AD treatment with DPP-4 inhibitors could also reduce the polypharmacy observed in elderly AD patients (45).
Furthermore, it has been reported that DPP-4 inhibitors may rescue striatal innervation of dopaminergic neurons in an animal model of Parkinson’s disease (PD) and inhibit motor neuron excitotoxic cell death in vitro (46). Consequently, DPP-4 inhibitors may also be effective towards the treatment of other neurodegenerative diseases.
Overall, a substantial growing body of evidence supports the use of DPP-4 inhibitors as a promising novel approach for AD treatment. Clearly, these encouraging findings emphasize the need for future observational studies with more participants and over longer periods of time, as well as randomized controlled trials for acquisition of clinically meaningful results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hamilton JM, Salmon DP, Raman R, et al. Accounting for functional loss in Alzheimer's disease and dementia with Lewy bodies: beyond cognition. Alzheimers Dement 2014;10:171-8. [Crossref] [PubMed]
- Moraes SR, Silva LS. An evaluation of the burden of Alzheimer patients on family caregivers. Cad Saude Publica 2009;25:1807-15. [Crossref] [PubMed]
- Grutzendler J, Morris JC. Cholinesterase inhibitors for Alzheimer's disease. Drugs 2001;61:41-52. [Crossref] [PubMed]
- Mohamed S, Rosenheck R, Lyketsos CG, et al. Caregiver burden in Alzheimer disease: cross-sectional and longitudinal patient correlates. Am J Geriatr Psychiatry 2010;18:917-27. [Crossref] [PubMed]
- Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med 2016;18:421-30. [Crossref] [PubMed]
- Kosaraju J, Gali CC, Khatwal RB, et al. Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer's disease. Neuropharmacology 2013;72:291-300. [Crossref] [PubMed]
- Isik AT, Soysal P, Yay A, et al. The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer's disease. Diabetes Res Clin Pract 2017;123:192-8. [Crossref] [PubMed]
- Tramutola A, Arena A, Cini C, et al. Modulation of GLP-1 signaling as a novel therapeutic approach in the treatment of Alzheimer's disease pathology. Expert Rev Neurother 2017;17:59-75. [Crossref] [PubMed]
- Yang Y, Song W. Molecular links between Alzheimer's disease and diabetes mellitus. Neuroscience 2013;250:140-50. [Crossref] [PubMed]
- Li X, Song D, Leng SX. Link between type 2 diabetes and Alzheimer's disease: from epidemiology to mechanism and treatment. Clin Interv Aging 2015;10:549-60. [Crossref] [PubMed]
- Hölscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol 2014;221:T31-41. [Crossref] [PubMed]
- D'Amico M, Di Filippo C, Marfella R, et al. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer's prone mice. Exp Gerontol 2010;45:202-7. [Crossref] [PubMed]
- Perry T, Greig NH. The glucagon-like peptides: a new genre in therapeutic targets for intervention in Alzheimer's disease. J Alzheimers Dis 2002;4:487-96. [Crossref] [PubMed]
- Salcedo I, Tweedie D, Li Y, et al. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol 2012;166:1586-99. [Crossref] [PubMed]
- Biswas SC, Buteau J, Greene LA. Glucagon-like peptide-1 (GLP-1) diminishes neuronal degeneration and death caused by NGF deprivation by suppressing Bim induction. Neurochem Res 2008;33:1845-51. [Crossref] [PubMed]
- Ahrén B, Schmitz O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res 2004;36:867-76. [Crossref] [PubMed]
- Hasan AA, Hocher B. Role of soluble and membrane-bound dipeptidyl peptidase-4 in diabetic nephropathy. J Mol Endocrinol 2017;59:R1-10. [Crossref] [PubMed]
- Kosaraju J, Holsinger RMD, Guo L, et al. Linagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Mitigates Cognitive Deficits and Pathology in the 3xTg-AD Mouse Model of Alzheimer's Disease. Mol Neurobiol 2017;54:6074-84. [Crossref] [PubMed]
- Kornelius E, Lin CL, Chang HH, et al. DPP-4 Inhibitor Linagliptin Attenuates Abeta-induced Cytotoxicity through Activation of AMPK in Neuronal Cells. CNS Neurosci Ther 2015;21:549-57. [Crossref] [PubMed]
- Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem 2008;104:1433-9. [Crossref] [PubMed]
- An FM, Chen S, Xu Z, et al. Glucagon-like peptide-1 regulates mitochondrial biogenesis and tau phosphorylation against advanced glycation end product-induced neuronal insult: Studies in vivo and in vitro. Neuroscience 2015;300:75-84. [Crossref] [PubMed]
- Wu YJ, Guo X, Li CJ, et al. Dipeptidyl peptidase-4 inhibitor, vildagliptin, inhibits pancreatic beta cell apoptosis in association with its effects suppressing endoplasmic reticulum stress in db/db mice. Metabolism 2015;64:226-35. [Crossref] [PubMed]
- Li Y, Guo Y, Tang J, et al. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 2014;46:629-40. [Crossref] [PubMed]
- Kröller-Schön S, Knorr M, Hausding M, et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res 2012;96:140-9. [Crossref] [PubMed]
- Kosaraju J, Murthy V, Khatwal RB, et al. Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer's disease. J Pharm Pharmacol 2013;65:1773-84. [Crossref] [PubMed]
- Kosaraju J, Madhunapantula SV, Chinni S, et al. Dipeptidyl peptidase-4 inhibition by Pterocarpus marsupium and Eugenia jambolana ameliorates streptozotocin induced Alzheimer's disease. Behav Brain Res 2014;267:55-65. [Crossref] [PubMed]
- Rizzo MR, Barbieri M, Boccardi V, et al. Dipeptidyl peptidase-4 inhibitors have protective effect on cognitive impairment in aged diabetic patients with mild cognitive impairment. J Gerontol A Biol Sci Med Sci 2014;69:1122-31. [Crossref] [PubMed]
- Qin Z, Sun Z, Huang J, et al. Mutated recombinant human glucagon-like peptide-1 protects SH-SY5Y cells from apoptosis induced by amyloid-beta peptide (1-42). Neurosci Lett 2008;444:217-21. [Crossref] [PubMed]
- Perry T, Lahiri DK, Sambamurti K, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res 2003;72:603-12. [Crossref] [PubMed]
- Sharoyan S, Antonyan A, Mardanyan S, et al. Interaction of dipeptydil peptidase IV with amyloid peptides. Neurochem Int 2013;62:1048-54. [Crossref] [PubMed]
- Piccini A, Russo C, Gliozzi A, et al. beta-amyloid is different in normal aging and in Alzheimer disease. J Biol Chem 2005;280:34186-92. [Crossref] [PubMed]
- Parihar MS, Brewer GJ. Amyloid-beta as a modulator of synaptic plasticity. J Alzheimers Dis 2010;22:741-63. [Crossref] [PubMed]
- Gengler S, McClean PL, McCurtin R, et al. Val(8)GLP-1 rescues synaptic plasticity and reduces dense core plaques in APP/PS1 mice. Neurobiol Aging 2012;33:265-76. [Crossref] [PubMed]
- Li L, Zhang ZF, Holscher C, et al. (Val(8)) glucagon-like peptide-1 prevents tau hyperphosphorylation, impairment of spatial learning and ultra-structural cellular damage induced by streptozotocin in rat brains. Eur J Pharmacol 2012;674:280-6. [Crossref] [PubMed]
- Li Y, Duffy KB, Ottinger MA, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis 2010;19:1205-19. [Crossref] [PubMed]
- Ji C, Xue GF, Li G, et al. Neuroprotective effects of glucose-dependent insulinotropic polypeptide in Alzheimer's disease. Rev Neurosci 2016;27:61-70. [Crossref] [PubMed]
- Wang Q, Xu Y, Chen JC, et al. Stromal cell-derived factor 1alpha decreases beta-amyloid deposition in Alzheimer's disease mouse model. Brain Res 2012;1459:15-26. [Crossref] [PubMed]
- Severini C, Petrella C, Calissano P. Substance P and Alzheimer's Disease: Emerging Novel Roles. Curr Alzheimer Res 2016;13:964-72. [Crossref] [PubMed]
- Duarte-Neves J, Pereira de Almeida L, Cavadas C., Neuropeptide Y. NPY) as a therapeutic target for neurodegenerative diseases. Neurobiol Dis 2016;95:210-24. [Crossref] [PubMed]
- Herskovits AZ, Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron 2014;81:471-83. [Crossref] [PubMed]
- Chong ZZ, Shang YC, Wang S, et al. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets 2012;16:167-78. [Crossref] [PubMed]
- Kumar R, Chaterjee P, Sharma PK, et al. Sirtuin1: a promising serum protein marker for early detection of Alzheimer's disease. PLoS One 2013;8. [Crossref] [PubMed]
- Folch J, Petrov D, Ettcheto M, et al. Current Research Therapeutic Strategies for Alzheimer's Disease Treatment. Neural Plast 2016;2016. [Crossref] [PubMed]
- Yang C, Xiao S. New developments of clinical trial in immunotherapy for Alzheimer's disease. Curr Pharm Biotechnol 2015;16:484-91. [Crossref] [PubMed]
- Kuo TC, Zhao Y, Weir S, et al. Implications of comorbidity on costs for patients with Alzheimer disease. Med Care 2008;46:839-46. [Crossref] [PubMed]
- Wu YQ, Limburg DC, Wilkinson DE, et al. Neuroprotective effects of inhibitors of dipeptidyl peptidase-iV in vitro and in vivo. Adv Exp Med Biol 2003;524:351-5. [Crossref] [PubMed]