The expression of KATP channel subunits in alpha-synuclein-transfected MES23.5 cells
Introduction
Parkinson’s disease (PD) is one of the leading neurodegenerative diseases in elderly, which is pathologically caused by the progressive loss of dopaminergic (DAergic) neurons in the substantia nigra (SN), and a formation of alpha-synuclein (α-Syn) aggregates in Lewy bodies throughout the brain. However, the mechanism of the selective DAergic neurons death is still unknown (1-3).
The mRNA levels of SUR1 subunit of KATP channels in the remaining DA neurons were increased in SN region of PD brains, and some studies have also addressed that the mRNA expression of SUR1 in the nigral DAergic neurons was approximately two-fold to that in the ventral tegmental area (VTA) in a PD animal model (4,5), suggesting that specific activation of the KATP channels in the SN might be related to the regression of DAergic neurons. KATP channels were first demonstrated in the mammalian heart cells (6). In brain, KATP channels are ubiquitously expressed, especially in the cortex, basal ganglia, hippocampus, hypothalamus, and nigral DAergic neurons (7,8). In nigral DAergic neurons, KATP channels are more sensitive to some mitochondrial complex I inhibitors, like rotenone or 1-Methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP) (9). KATP channels consist of Kir6.x (Kir6.1 or Kir6.2) subunits and SUR (SUR1 or SUR2) subunits (10,11). Three subtypes of KATP channels, SUR1, SUR2B, and Kir6.2, have been identified in the midbrain DAergic neurons (12). Of these, SUR1/Kir6.2 subtype of KATP channels were extremely sensitive to mitochondrial complex I blockers, which is about 200 times as big as SUR2B/Kir6.2 subtype of KATP channels (13).
This study examines the expressions of KATP channel subunits in α-Syn-transfected cells. The mRNA levels of SUR1 but not SUR2B or Kir6.2 were selectively upregulated in these cells.
Methods
Reagents
The MES23.5 cells were obtained from Stem Cell Bank (Chinese Academy of Sciences). The GV230 vectors that carrying cDNA encoding WT or A53T α-Syn were purchased from GeneChem Co., Ltd. (Shanghai, China). Fetal Bovine Serum (FBS), Trizol Reagent and Lipofectamine 2000 were purchased from Invitrogen (CA, USA). Dulbecco’s modified Eagle’s medium (DMEM) and other chemical drugs were obtained from Sigma (St. Louis, MO, USA).
Cell culture
The MES23.5 cell, a DAergic cell line, is obtained from the murine neuroblastoma glioma N18TG2 cells hybridized with the rat mesencephalic neurons. It owns a number of properties which are resemble to the DAergic neurons from the SN. MES23.5 cells were maintained in DMEM which contained FBS (10%), Sato’s (2%), penicillin (100 U/mL) and streptomycin (100 U/mL) in a humidified atmosphere containing 5% CO2 at 37 °C, and seeded into the 6 wells plastic plates (1×105 cells per well).
Cell transfection
To observe the expressions of distinct KATP channel subunits, the cells were separated to three groups: GV230 vector group, WT α-Syn group and A53T α-Syn group. In the control group, GV230 vectors were transfected into MES23.5 cells alone. In the over-expression group, cells were transfected with the WT or A53T α-Syn in a serum-free medium. This relatively high efficiency of infection of MES23.5 cells were harvested for the further studies. All vectors were transfected using Lipofectamine 2000. For optimal transfection efficiency, five volume of DNA relative to lipofectamine 2000 was used.
Total RNA extraction and real-time quantitative PCR
Total RNA was harvested using Trizol Reagent based on the manufacturer’s protocols. A total of 2 µg RNA was reversely transcribed into cDNA using a First Strand cDNA Synthetic Kit. The mRNA levels of KATP channel subunits were detected using quantitative PCR with SYBR Green reagents. The primer sequences used as follows: SUR1 (forward: 5'-CCC TAG CTG TGG TGT GCT ACT TCA-3'; reverse: 5'-GGG GCT GCG TTG TGT CATC-3'); and SUR2B (forward: 5'-TGG AGC TGA CAG ACA CGA ACA AC-3'; reverse: 5'-GAA CAA TGC ACG CTC CCA GA-3'); and Kir6.2 (forward: 5'-ATG GCC CTG ACA GGC AAG AG-3'; reverse: 5'-CCA AGT TGG CCA GAC AGA CAG A-3'). GAPDH purchased from Takara was served as a normalization control. The amplification was carried out according to the following steps: the mixture was preheated at 95 °C for 30 s, followed by 5 s, 95 °C and 34 s, 60 °C for total 40 cycles. Relative mRNA levels were calculated by the 2−ΔΔCt method.
Statistical analysis
All data were presented as mean ± SEM. Statistical analysis was performed using Graphpad 5.0. One-way analysis of variance (ANOVA) with Tukey’s multiple comparison tests were carried out for multiple comparisons. Values of P<0.05 were deemed to be significant.
Results
Increased SUR1 mRNA levels were observed after α-Syn transfection
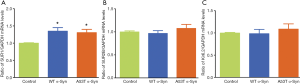
We detected the expressions of distinct subunits of KATP channels in transfected MES23.5 cells with WT or A53T α-Syn for 24 hrs. The results illustrated that the mRNA levels of SUR1 were increased by 35% and by 31%, respectively, when were compared with that in the control (Figure 1A). SUR2B and Kir6.2 were slightly increased in A53T α-Syn group, but there were no significances observed (Figure 1B,C).
Discussion
In our study, the mRNA levels of SUR1 were found increased in either WT α-syn or A53T α-Syn transfected MES23.5 cells, while there were no effects on Kir6.2 and SUR2B transcriptions.
Though the precise mechanisms underlying the PD pathology are still unknown, some evidence has shown that selective activation of SUR1/Kir6.2 subtype of KATP channels in SN may lead to the degeneration of DA neurons. The transcript levels of SUR1 subunit of KATP channels in the remaining DA neurons were increased in SN of PD brains (4). The increased SUR1 subunit expression could promote the transport, expression and activation of KATP channel in membrane (14,15). Previously, our studies had reported that the activation of KATP channels enhanced iron uptake mediated by divalent metal transporter 1 (DMT1) in SK-N-SH cells, implicating an important role of KATP channels on the cell membrane hyperpolarization. Furthermore, a decrease in ratio of ATP/ADP and an increase in the production of ROS induce additional KATP channels activation in a feed-forward cycle (16). In both PD patients and animal models, neuroscientists found the SUR1 subunit of KATP channels was highly expressed in the remaining nigral DAergic neurons, and the SUR1 subunit was involved in KATP channels trafficking to the cell membrane (4,5). An increase in the number of functional KATP channels which triggered burst firing might compensate the loss of nigrostriatal dopamine-induced by the progressive degenerations of DAergic neurons (17). Nevertheless, the constant burst firing of DAergic neurons triggered by KATP channels could lead to excitotoxicity, elevate calcium loading, reduce calcium buffering capacities, cause the ROS production, and a variety of processes which result in detrimental effect to DAergic neurons in a long term (18-20).
In pancreatic β-cells, SUR1 expressions are regulated by many factors, such as Hsp90, insulin, PKA, FOXA1 and FOXA2 (21-23). FOXA1 and FOXA2 are so-called “pioneer proteins”, which could facilitate access of other transcription factors by binding to condensed chromatin in promoters and enhancer regions tightly (24). FOXA1 and FOXA2 are members of the winged-helix/fork head transcription factors which play a major role in the development of midbrain DA neurons during the early and late embryonic period (25-27). Moreover, FOXA1 and FOXA2 are also necessary for the maintenance of appropriate firing patterns of SN pars compacta neurons. It has been showed that the burst firing of nigral DAergic neurons is reduced in response to the deletion of FOXA1 and FOXA2 (28). FOXA1 and FOXA2 may regulate the expression of SUR1 subunit of KATP channels in DA neurons.
In conclusion, we showed that the mRNA levels of SUR1 but not SUR2B or Kir6.2 were selectively upregulated in MES23.5 cells over-expressed α-Syn. Therefore, the present study could provide a new evidence for the influence of KATP channels in the loss of DAergic neurons in PD.
Acknowledgements
Funding: Our study was supported by grants from the National Foundation of Natural Science of China [31701020], Shandong Provincial Education Department (J15LE18), China postdoctoral science foundation (2016M602099). Clinical+X foundation of the medical college of Qingdao University (2017M33, 2017Q57).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson's disease pathology and genetics. Nature 2016;539:207-16. [Crossref] [PubMed]
- Lu M, Zhao FF, Tang JJ, et al. The neuroprotection of hydrogen sulfide against MPTP-induced dopaminergic neuron degeneration involves uncoupling protein 2 rather than ATP-sensitive potassium channels. Antioxid Redox Signal 2012;17:849-59. [Crossref] [PubMed]
- Eschbach J, Danzer KM. alpha-Synuclein in Parkinson's disease: pathogenic function and translation into animal models. Neurodegener Dis 2014;14:1-17. [Crossref] [PubMed]
- Schiemann J, Schlaudraff F, Klose V, et al. K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat Neurosci 2012;15:1272-80. [Crossref] [PubMed]
- Liss B, Haeckel O, Wildmann J, et al. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci 2005;8:1742-51. [Crossref] [PubMed]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 1983;305:147-8. [Crossref] [PubMed]
- Dunn-Meynell AA, Routh VH, McArdle JJ, et al. Low-affinity sulfonylurea binding sites reside on neuronal cell bodies in the brain. Brain Res 1997;745:1-9. [Crossref] [PubMed]
- Liss B, Bruns R, Roeper J. Alternative sulfonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurons. Embo J 1999;18:833-46. [Crossref] [PubMed]
- Roper J, Ashcroft FM. Metabolic inhibition and low internal ATP activate K-ATP channels in rat dopaminergic substantia nigra neurones. Pflugers Arch 1995;430:44-54. [Crossref] [PubMed]
- Vedovato N, Ashcroft FM, Puljung MC. The Nucleotide-Binding Sites of SUR1: A Mechanistic Model. Biophys J 2015;109:2452-60. [Crossref] [PubMed]
- Li N, Wu JX, Ding D, et al. Structure of a Pancreatic ATP-Sensitive Potassium Channel. Cell 2017;168:101-10.e10. [Crossref] [PubMed]
- Yamada M, Isomoto S, Matsumoto S, et al. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol 1997;499:715-20. [Crossref] [PubMed]
- Adebiyi A, McNally EM, Jaggar JH. Sulfonylurea receptor-dependent and -independent pathways mediate vasodilation induced by ATP-sensitive K+ channel openers. Mol Pharmacol 2008;74:736-43. [Crossref] [PubMed]
- Sharma N, Crane A, Clement JP, et al. The C terminus of SUR1 is required for trafficking of KATP channels. J Biol Chem 1999;274:20628-32. [Crossref] [PubMed]
- Martin GM, Chen PC, Devaraneni P, et al. Pharmacological rescue of trafficking-impaired ATP-sensitive potassium channels. Front Physiol 2013;4:386. [Crossref] [PubMed]
- Du X, Xu H, Shi L, et al. Activation of ATP-sensitive potassium channels enhances DMT1-mediated iron uptake in SK-N-SH cells in vitro. Sci Rep 2016;6:33674. [Crossref] [PubMed]
- Nichols CG. K. ATP channels as molecular sensors of cellular metabolism. Nature 2006;440:470-6. [Crossref] [PubMed]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993;262:689-95. [Crossref] [PubMed]
- Beal MF. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann Neurol 1998;44:S110-4. [Crossref] [PubMed]
- Krieger C, Duchen MR. Mitochondria, Ca2+ and neurodegenerative disease. Eur J Pharmacol 2002;447:177-88. [Crossref] [PubMed]
- Yan FF, Pratt EB, Chen PC, et al. Role of Hsp90 in biogenesis of the beta-cell ATP-sensitive potassium channel complex. Mol Biol Cell 2010;21:1945-54. [Crossref] [PubMed]
- Chen PC, Kryukova YN, Shyng SL. Leptin regulates KATP channel trafficking in pancreatic beta-cells by a signaling mechanism involving AMP-activated protein kinase (AMPK) and cAMP-dependent protein kinase (PKA). J Biol Chem 2013;288:34098-109. [Crossref] [PubMed]
- Heddad Masson M, Poisson C, Guerardel A, et al. Foxa1 and Foxa2 regulate alpha-cell differentiation, glucagon biosynthesis, and secretion. Endocrinology 2014;155:3781-92. [Crossref] [PubMed]
- Cirillo LA, Lin FR, Cuesta I, et al. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell 2002;9:279-89. [Crossref] [PubMed]
- Ang SL. Foxa1 and Foxa2 transcription factors regulate differentiation of midbrain dopaminergic neurons. Adv Exp Med Biol 2009;651:58-65. [Crossref] [PubMed]
- Lin W, Metzakopian E, Mavromatakis YE, et al. Foxa1 and Foxa2 function both upstream of and cooperatively with Lmx1a and Lmx1b in a feedforward loop promoting mesodiencephalic dopaminergic neuron development. Dev Biol 2009;333:386-96. [Crossref] [PubMed]
- Jackson BC, Carpenter C, Nebert DW, et al. Update of human and mouse forkhead box (FOX) gene families. Hum Genomics 2010;4:345-52. [PubMed]
- Pristera A, Lin W, Kaufmann AK, et al. Transcription factors FOXA1 and FOXA2 maintain dopaminergic neuronal properties and control feeding behavior in adult mice. Proc Natl Acad Sci U S A 2015;112:E4929-38. [Crossref] [PubMed]


