Heart-lung interactions in acute respiratory distress syndrome: pathophysiology, detection and management strategies
Introduction
The acute respiratory distress syndrome (ARDS) is the most severe form of acute respiratory failure still linked to a high morbidity and mortality (1). One of the major advances in the management of ARDS has been the introduction of lung protective ventilation strategies which can be considered the first therapeutic intervention consistently improving outcomes (2,3). Based on the strict limitation of tidal volumes and inspiratory alveolar pressures these strategies aim at preventing ventilation induced lung injury (VILI) by minimizing tidal overdistension and recruitment (4). However, the heterogenous nature of the parenchymal lung involvement creates important regional differences that greatly amplify lung tissue strain that can trigger an inflammatory response in those areas receiving a disproportionately high local tidal volume (5). The ventilator management of ARDS has mainly focused on preventing the deleterious effects of mechanical ventilation on the alveolar compartment. Surprisingly, the effects on the vascular spaces have received much less attention, even though the pathophysiological involvement of the microcirculation and its effect on pulmonary hemodynamics and right ventricular (RV) function have been known for long (6-8). Furthermore, the close link between the airway and vascular spaces in the pathophysiology of VILI has been well established experimentally and is referred to as vascular VILI (9). Recently, a unifying parameter, the inspiratory driving pressure, has been identified as the single most relevant independent factor mediating outcome in ARDS (10). This easy to measure parameter, obtained by substracting total PEEP from plateau pressure, is closely related to the cyclic tidal strain imposed on the functional ventilated lung. Being an expression of the ratio between the delivered tidal volume and lung compliance, driving pressure integrates information on the effects of tidal volume, plateau pressure and PEEP. An elevated driving pressure is not only a risk factor for damaging the alveolar compartment but has also been recognized as the main independent ventilatory factor causing PVD (11).
In this report, we will discuss key pathophysiological aspects of the interaction between the lung, the pulmonary circulation and the right heart and discuss management strategies to prevent the negative impact of mechanical ventilation on this interaction in ARDS.
Pulmonary vascular dysfunction (PVD)
The pulmonary vascular involvement in ARDS was first described more than 40 years ago (6). Now referred to as PVD, this term encompasses the structural and functional pathophysiological changes affecting the vascular compartment and the RV in ARDS. These changes include pulmonary vasoconstriction induced by hypoxia and/or the release of vasoactive inflammatory mediators, microvascular thrombosis, reduction in functional lung volume, direct inflammatory endothelial damage and vascular remodeling phenomena (12). The clinical expression of PVD is an increase in pulmonary arterial (PA) pressure and vascular resistance (PVR). It should be considered as a continuum during the course of ARDS ranging from mild pulmonary hypertension, invariably present in most ARDS patients, to more severe pulmonary hypertension and ultimately RV failure (Figure 1). Although not unequivocally established (13) accumulating clinical data strongly suggest a prognostic link between PVD and outcome in ARDS (11,14). Furthermore, an elevated physiological dead-space, an indirect marker of PVD as it reflects early microvascular alterations, has been a consistent independent predictor of ARDS mortality (15,16). In nearly 25% of patients PVD evolves to its most severe form acute cor-pulmonale with overt RV failure (17) in which the reported associated mortality has been as high as 60–70% (11).
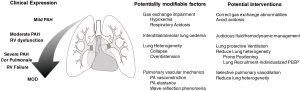
Pathophysiological aspects of pulmonary and RV hemodynamics
The effects of mechanical ventilation
The pulmonary circulation is a low pressure—low resistance circuit that together with the RV and the lung shares a common physical space in the thoracic cavity. Their operation and interaction are thus influenced by the pressure level surrounding these structures, the pleural pressure, and the cyclic changes in the lung distending pressure, the transpulmonary pressure (i.e., the alveolar minus pleural pressure). During normal breathing pleural and transpulmonary pressure swings are of very low magnitude, slightly above and below atmospheric levels minimizing lung stress, facilitating venous return and optimizing ventilation and perfusion matching. This balance is profoundly affected during positive pressure ventilation. The operating pressure is increased to a continuous level, the positive end-expiratory pressure (PEEP) which is transmitted to the right atrium directly reducing the pressure gradient for venous return. In addition, transpulmonary pressure swings above PEEP become significantly higher affecting RV afterload. Both, RV and pulmonary circulation can normally adapt to these changes. However, under pathological conditions such as ARDS, where higher than normal pressures are applied in a heterogeneously diseased lung, this adaptation capacity is critically challenged.
Pulmonary vascular mechanics
To accommodate the entire output of the RV, normal pulmonary vessels are highly distensible offering a low resistance to flow. This low PA elastance is essential to maintain RV efficiency, that is, the effective transfer of power from the ejecting ventricle to the pulmonary circulation at the lowest energetic cost. This critical dependency between RV and pulmonary vascular mechanics is represented by the concept of ventricular-vascular coupling (18). In patients with pulmonary hypertension, the increase in proximal arterial elastance, described as arterial stiffness, is believed to mark the beginning of the transition from a compensated to a non-compensated RV dysfunction and is a strong predictor of mortality (19,20). Increased proximal arterial elastance may also be an important early component of PVD during ARDS due to the predominant vasoconstrictive state caused by hypoxemia, hypercapnic and metabolic acidosis, vasoactive mediators and the reduction of functional lung volume (Figure 1). Most of these factors primarily affect medial and distal PA segments but result in dilation of proximal segments that become stiffer (21). It has been established that this increase in PA elastance is probably as important as an elevated PVR in increasing RV load (22). Another important but less studied factor that affects ventricular-vascular coupling is related to wave reflection phenomena. Ventricular systole generates a pressure and flow wave that travels through the pulmonary circulation and is reflected in the distally branching arterial tree. When the forward pressure and flow waves generated during RV systole meet the backward returning wave, pressure is increased and flow decreased. In the normal pulmonary circulation wave reflection phenomena are negligible as reflected waves arrive during the diastolic period. However, in pathological conditions with increased PA elastance and resistance, reflected waves are regularly present travel faster and thus arrive earlier during systole affecting RV ejection and efficiency (23). The site of the reflected wave arrival can often be seen by a notching in the ejective phase of the systolic portion of the PA waveform and the pressure increase above this point is the amplified load that the RV has to overcome. Pulmonary wave reflections have been documented in patients with pulmonary hypertension by time domain analysis of the pulmonary artery pressure waveform (24) but have not been evaluated in ARDS patients. In a recent experimental ARDS study PA wave reflections had an important contribution to RV afterload and could be modulated by the lung condition (23) (Figure 2).
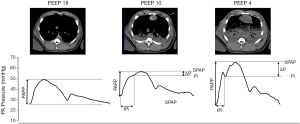
Clinical assessment of pulmonary hemodynamics and RV function
Pulmonary artery catheter
One of the reasons of the reduced attention devoted to PVD in ARDS may be related to the intrinsic difficulty to properly assess pulmonary circulation and RV function at the bedside. The pulmonary artery catheter was essential in the detection and first description of PVD (6) but, its current clinical use has declined significantly. It provides a direct continuous monitoring of pulmonary artery pressure waveforms and it measures PA occlusion pressure and RV cardiac output by thermodilution. This allows to calculate pulmonary vascular resistance (PVR), i.e., the ratio of mean transpulmonary arterial pressure drop to cardiac output, the main clinical estimate of RV afterload. By using mean values however, PVR represents only the steady-state opposition to ventricular output neglecting the pulsatile nature of pulmonary flow and pressure which can significantly contribute to RV load. The calculation of PVR is also based on assumptions, such as that flow occurs through a rigid tube without branching and ignores the role of inertance and blood viscosity, that are not met in the pulmonary circulation. In fact, RV afterload is a much more complex concept that should include all the factors that oppose efficient ventricular ejection including vascular stiffness and ventricular-vascular coupling phenomena that better describe the dynamically changing conditions in which the RV must operate. More specific methods to assess total RV load and pulmonary circulation such as PA impedance and its components, arterial elastance and advanced instantaneous flow and pressure waveform analysis would be needed to more comprehensively assess RV afterload. Although such methods have provided very useful mechanistic insights in the pathophysiology of heart-lung interactions they are not available for clinical use.
Echocardiography
Echocardiography has become a very useful first line bedside diagnostic tool to assess RV function (25) and to detect the presence of PVD (17). It informs about RV dimensions and function and can provide estimates of RV outflow, PA pressure and assess preload dependency. It also evaluates parameters that describe the interaction between RV and pulmonary circulation such as an estimate of PVR (26), RV ejection efficiency (27) and speckle tracking (28). Echocardiography provides the currently accepted diagnostic criteria for moderate PVD: pulmonary artery systolic pressure >40 mmHg and severe PVD: RV to left ventricular end-diastolic area ratio <0.6 and end-systolic paradoxical septum motion (11). However, echocardiographic assessment of PVD is not always easy due to the complex RV shape and the quality of the acoustic window often compromised in ventilated patients. In addition, echocardiography only offers intermittent evaluations precluding the longitudinal assessment over time and during specific interventions or changing conditions.
Management strategies to improve heart-lung interactions in ARDS
Whether the clinical expression of PVD remains as a mild pulmonary hypertension or evolves to acute cor-pulmonale depends on the extent of the intrinsic vascular involvement related to ARDS, but also on the clinical and ventilatory management. General therapeutic interventions such as the specific treatment of the precipitating cause of ARDS, adequate hemodynamic management with judicious fluid administration, especially in the post-resuscitation phase and correction of gas exchange abnormalities are mandatory. Implementing therapeutic strategies directly aimed at improving or reversing factors related to the development of PVD would be of particular interest (Figure 1).
Pulmonary vasodilators
Selective pulmonary vasodilators such as inhaled nitric oxide (iNO), prostacyclin and epoprostenol are attractive therapeutic options for PVD as they directly reduce PA pressure and PVR without systemic hemodynamic effects (29). Data on the most studied agent, iNO are inconclusive and clinical trials have not demonstrated any outcome benefit (30). However, the study protocols tested to date have not been specifically targeted to improve PVD which may require different doses and therapeutic strategies. Interestingly iNO is still a commonly used rescue therapy in ARDS patients (31). The role of other vasodilators such as phosphodiesterase-5 inhibitors or endothelin-receptor antagonists in the treatment of PVD in ARDS is not known.
Lung protective ventilation strategies
The role of mechanical ventilation is of particular interest as it constitutes a potentially modifiable factor that, with the appropriate settings, can modulate heart-lung interactions. The use of high tidal volumes, plateau and driving pressures have shown to directly cause (11,32) or worsen PVD (33). Lung protective ventilation strategies have therefore an important role in the prevention of the occurrence and progression of PVD and RV failure (34). Recently described RV protective ventilation strategies recommend the limitation of plateau and driving pressures by using low tidal volumes and moderate levels of PEEP (35). The importance of functional lung volume is also increasingly recognized and is well explained by the u-shaped relation between lung volume and PVR. At high lung volumes compression of alveolar vessels directly increases PVR. At low volumes the lung tends to collapse triggering hypoxic pulmonary vasoconstriction, affecting extra-alveolar vessels’ geometry and causing capillary de-recruitment. Collapse also increases lung heterogeneity and reduces the size of the functional lung that participates in tidal ventilation. In this condition, and depending on the amount of lung collapse which can be extensive in ARDS (36,37), even recommended lower tidal volumes can cause significant lung overdistension (38) with obvious negative consequences for both air and vascular spaces. This may explain, at least in part, why the reported incidence of PVD in the era of lung protective ventilation is still high (11,14).
The role of PEEP
During mechanical ventilation lung volume is critically affected by the level of PEEP. In ARDS lungs normally aerated and non-dependent overinflated lung regions often coexist with dependent lung collapse. In such conditions, the theoretical beneficial effects of lower PEEP on pulmonary circulation and RV function can be offset by the negative consequences of collapse whereas high levels can be either beneficial when promoting recruitment or detrimental when contributing to further overdistension. In other words, how PEEP affects lung volume is a major determinant of its effects on afterload (Figure 2). A good example illustrating this comes from a recent study on the prevalence of PVD in which patients developing acute cor pulmonale were in fact ventilated with lower levels of PEEP (11).
Prone positioning
One effective way to reduce lung heterogeneity is prone positioning. By modulating the vertical pleural pressure gradient, proning homogenizes the regional distribution of transpulmonary pressures reexpanding dependent lung regions (39). This increase in functional lung volume by recruitment may well be one of the main mechanisms behind the described RV unloading effects of prone positioning (40,41).
In a recent randomized controlled trial in severe ARDS patients lung protective ventilation in the prone position resulted in a significant improvement in survival and interestingly in less cardiovascular dysfunction (42). Proning also usually results in better oxygenation and carbon dioxide elimination, which per se contributes to RV unloading.
The open lung approach (OLA)
A potentially more efficient way to reduce lung heterogeneity and prevent PVD is the OLA ventilation strategy. It aims at restoring the functional size of the lung by combining lung recruitment with an individualized selection of PEEP. Beneficial effects on PVD of such a strategy have been recently demonstrated in patients submitted to cardiac surgery (43). Recruitment immediately after bypass weaning resulted in improved RV function assessed by echocardiography. Figure 3 illustrates the effects of recruitment on PA pressures, pulsatility and waveform morphology in a cardiac surgery patient. Key to the OLA strategy is the appropriate individual selection of PEEP after lung recruitment. By using clinical parameters such as dynamic compliance a decremental PEEP trial aims at finding the minimum level i.e., the open lung PEEP that prevents lung collapse (44,45). By maintaining this level, ventilation can then be set at the minimum possible driving pressure to minimize tidal overdistension. When successfully applied in a responding patient this strategy places tidal ventilation at the nadir of the PVR-lung volume relationship and the individualized PEEP level represents the best compromise between lung collapse and overdistension. Open lung PEEP levels frequently reach initial values of 15 to 17 cmH2O that in a recent randomized controlled trial were not associated with negative hemodynamic effects when compared with the standard ARDSnet strategy (45). The effects of open lung PEEP on PVD has been recently evaluated in an experimental ARDS model by advanced analysis of pulmonary vascular mechanics. Open lung PEEP levels averaged 19 cmH2O and resulted in less wave refection phenomena and a lower PA effective elastance without affecting oxygen delivery and systemic hemodynamics when compared to 10 cmH2O PEEP a level commonly used in ARDS patients (23).
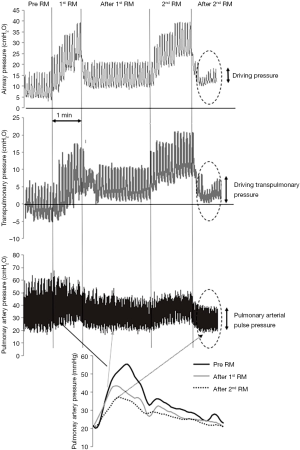
Conclusions
ARDS is still associated to a high mortality and the importance of pulmonary vascular and RV involvement in its pathophysiology and prognosis is increasingly recognized. The way the heart and the lungs interact during mechanical ventilation in ARDS is complex and difficult to evaluate at the bedside. Classic hemodynamic assessment such as PVR has important limitations as RV afterload is dynamically affected by many other factors related to pulmonary vascular mechanics and ventricular vascular coupling. Better understanding the complex pathophysiology governing heart lung interactions during the mechanical ventilatory support of ARDS is essential to adequately implement therapeutic interventions to prevent the occurrence and progression of both pulmonary and vascular dysfunction. The adoption of these therapeutic interventions should be considered early in the course of ARDS in order to impact outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017;377:562-72. [Crossref] [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Borges JB, Costa EL, Suarez Sipmann F, et al. Early inflammation mainly affects normally and poorly aerated lung in experimental ventilator-induced lung injury. Crit Care Med 2014;42:e279-87. [Crossref] [PubMed]
- Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med 1977;296:476-80. [Crossref] [PubMed]
- Villar J, Blazquez MA, Lubillo S, et al. Pulmonary hypertension in acute respiratory failure. Crit Care Med 1989;17:523-6. [Crossref] [PubMed]
- Squara P, Dhainaut JF, Artigas A, et al. Hemodynamic profile in severe ARDS: results of the European Collaborative ARDS Study. Intensive Care Med 1998;24:1018-28. [Crossref] [PubMed]
- Marini JJ, Hotchkiss JR, Broccard AF. Bench-to-bedside review: microvascular and airspace linkage in ventilator-induced lung injury. Crit Care 2003;7:435-44. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med 2013;39:1725-33. [Crossref] [PubMed]
- Price LC, McAuley DF, Marino PS, et al. Pathophysiology of pulmonary hypertension in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2012;302:L803-15. [Crossref] [PubMed]
- Ryan D, Frohlich S, McLoughlin P. Pulmonary vascular dysfunction in ARDS. Ann Intensive Care 2014;4:28. [Crossref] [PubMed]
- Bull TM, Clark B, McFann K, et al. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med 2010;182:1123-8. [Crossref] [PubMed]
- Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002;346:1281-6. [Crossref] [PubMed]
- Matthay MA, Kallet RH. Prognostic value of pulmonary dead space in patients with the acute respiratory distress syndrome. Crit Care 2011;15:185. [Crossref] [PubMed]
- Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med 2001;29:1551-5. [Crossref] [PubMed]
- Kussmaul WG, Noordergraaf A, Laskey WK. Right ventricular-pulmonary arterial interactions. Ann Biomed Eng 1992;20:63-80. [Crossref] [PubMed]
- Gan CT, Lankhaar JW, Westerhof N, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 2007;132:1906-12. [Crossref] [PubMed]
- Wang Z, Chesler N. Pulmonary vascular wall stiffness: an important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ 2011;1:212-23. [Crossref] [PubMed]
- al-Tinawi A, Krenz GS, Rickaby DA, et al. Influence of hypoxia and serotonin on small pulmonary vessels. J Appl Physiol 1994;76:56-64. [Crossref] [PubMed]
- Milnor WR, Conti CR, Lewis KB, et al. Pulmonary arterial pulse wave velocity and impedance in man. Circ Res 1969;25:637-49. [Crossref] [PubMed]
- Santos A, Lucchetta L, Monge-Garcia MI, et al. The open lung approach improves pulmonary vascular mechanics in an experimental model of acute respiratory distress syndrome. Crit Care Med 2017;45:e298-305. [Crossref] [PubMed]
- Castelain V, Hervé P, Lecarpentier Y, et al. Pulmonary artery pulse pressure and wave reflection in chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol 2001;37:1085-92. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography: Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. [Crossref] [PubMed]
- Abbas AE, Fortuin FD, Schiller NB, et al. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 2003;41:1021-7. [Crossref] [PubMed]
- López-Candales A, Lopez FR, Trivedi S, et al. Right ventricular ejection efficiency: a new echocardiographic measure of mechanical performance in chronic pulmonary hypertension. Echocardiography 2014;31:516-23. [Crossref] [PubMed]
- Orde SR, Behfar A, Stalboerger PG, et al. Effect of positive end-expiratory pressure on porcine right ventricle function assessed by speckle tracking echocardiography. BMC Anesthesiol 2015;15:49. [Crossref] [PubMed]
- Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J 2003;21:720-7. [Crossref] [PubMed]
- Adhikari NK, Burns KEA, Friedrich JO, et al. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ 2007;334:779. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Menendez C, Martinez-Caro L, Moreno L, et al. Pulmonary vascular dysfunction induced by high tidal volume mechanical ventilation. Crit Care Med 2013;41:e149-55. [Crossref] [PubMed]
- Vieillard-Baron A, Loubieres Y, Schmitt JM, et al. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol 1999;87:1644-50. [Crossref] [PubMed]
- Bouferrache K, Vieillard-Baron A. Acute respiratory distress syndrome, mechanical ventilation, and right ventricular function. Curr Opin Crit Care 2011;17:30-5. [Crossref] [PubMed]
- Vieillard-Baron A, Matthay M, Teboul JL, et al. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 2016;42:739-49. [Crossref] [PubMed]
- Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006;354:1775-86. [Crossref] [PubMed]
- de Matos GF, Stanzani F, Passos RH, et al. How large is the lung recruitability in early acute respiratory distress syndrome: a prospective case series of patients monitored by computed tomography. Crit Care 2012;16:R4. [Crossref] [PubMed]
- Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2007;175:160-6. [Crossref] [PubMed]
- Galiatsou E, Kostanti E, Svarna E, et al. Prone position augments recruitment and prevents alveolar overinflation in acute lung injury. Am J Respir Crit Care Med 2006;174:187-97. [Crossref] [PubMed]
- Vieillard-Baron A, Charron C, Caille V, et al. Prone Positioning Unloads the Right Ventricle in Severe ARDS* [Internet]. 2007; 132:1440-1446. Available online: http://journal.publications.chestnet.org/article.aspx?doi=10.1378/chest.07-1013
- Jozwiak M, Teboul JL, Anguel N, et al. Beneficial hemodynamic effects of prone positioning in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2013;188:1428-33. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Longo S, Siri J, Acosta C, et al. Lung recruitment improves right ventricular performance after cardiopulmonary bypass. Eur J Anaesthesiol 2017;34:66-74. [Crossref] [PubMed]
- Suarez-Sipmann F, Böhm SH, Tusman G, et al. Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit Care Med 2007;35:214-21. [Crossref] [PubMed]
- Kacmarek RM, Villar J, Sulemanji D, et al. Open lung approach for the acute respiratory distress syndrome. Crit Care Med 2016;44:32-42. [Crossref] [PubMed]


