Extracorporeal carbon dioxide removal in acute exacerbations of chronic obstructive pulmonary disease
Introduction
Extracorporeal carbon dioxide removal (ECCO2R) has been proposed as an adjunctive intervention to avoid worsening respiratory acidosis, thereby preventing or shortening the duration of invasive mechanical ventilation (IMV) in patients with exacerbation of chronic obstructive pulmonary disease (COPD). Despite being highly efficient from a physiological perspective, high-quality data assessing the risk-benefit ratio of this technology in patients with COPD are lacking. The present review summarizes the current evidence for the use of ECCO2R, with a particular focus on the pathophysiological rationale and clinical experience in patients with COPD.
Severe COPD exacerbations
Definition and epidemiology
COPD is a common, preventable, and treatable disease characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities, usually caused by significant exposure to noxious particles or gases (1). It is a major source of morbidity, mortality, and healthcare costs in the Western world (2-4), and it is projected to be the third leading cause of death worldwide in 2020 (5).
The natural course of the disease consists of periods of clinical stability interrupted by episodes of acute worsening of respiratory symptoms that result in additional therapy, termed exacerbations (1). COPD exacerbations negatively impact health status, rates of hospitalization, and disease progression: in particular, inpatient mortality from exacerbation range from 4% to 30% (6) and 5-year mortality rate after hospitalization is about 50% (7).
Pathophysiology of COPD exacerbations
Expiratory flow limitation caused by the increased resistance of the small airways is the pathophysiological hallmark of COPD and worsens during exacerbations. The increase airway resistance is associated with the increase of the expiratory time constant, which defines the time of exponential decrease of the lung volume during passive exhalation (8). The direct consequence of a longer time constant is the development of dynamic alveolar hyperinflation, which is the excessive increase in end-expiratory lung volume above the relaxation volume of the respiratory system, generating intrinsic positive end-expiratory pressure (8,9).
The acute worsening of dynamic hyperinflation during COPD exacerbations causes several detrimental effects on respiratory mechanics, muscle efficiency, alveolar ventilation/perfusion matching, and cardiovascular stability, leading to gas exchange abnormalities (9-11). In particular, hypercapnia can be severe and correlates with the risk of short and long-term mortality (10,12).
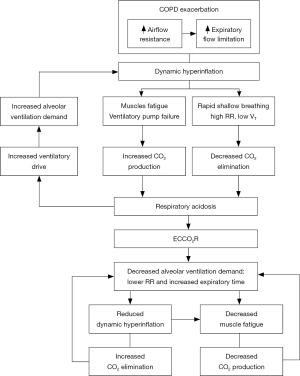
Ventilatory muscles adaptation to the chronic increase in resistive, elastic, and threshold loading may be quickly overwhelmed in the setting of increased dynamic hyperinflation (10), resulting in worsening hypercapnia and possibly decreased cardiac output. In the most severe cases, a vicious cycle arises that results from the increased ventilatory drive due to hypoxemia and carbon dioxide (CO2) retention, anxiety, fever, and increased sympathetic activation: this mechanism promotes further increase in lung elastance, dynamic hyperinflation, and muscle fatigue (10,13) (Figure 1).
Management of COPD exacerbations
Although preventing the development of acute events is crucial, when COPD exacerbations do occur, several pharmacological therapies are available (1). Non-invasive ventilation (NIV) is recognized as the gold standard in the management of COPD exacerbations with hypercapnic respiratory failure (1,14), as it improves gas exchange and work of breathing, and reduces the need for endotracheal intubation and mortality (15-22).
However, NIV carries a considerable failure rate estimated to be between 5–40% of COPD patients (14,21,23,24). Patients requiring the transition to IMV due to NIV failure are at significantly higher risk of death than those treated with NIV alone or with IMV from the outset (14). In a recent multicenter observational investigation of 3,520 patients with COPD exacerbation, NIV failure occurred in 14% of patients (22). Hospital mortality was 7% for patients treated with NIV; 16% for those treated with IMV; and 23% for those who failed NIV. Indeed, tracheal intubation and IMV have several detrimental side effects that may concur to determine high morbidity and mortality, such as the increased risk for ventilator-associated complications, tracheostomy and prolonged ventilation, and the need for sedation. Overall, these data suggest that avoiding IMV or shortening its duration would considerably improve outcomes in severe COPD exacerbations.
ECCO2R in COPD exacerbations
ECCO2R physiological principles
ECCO2R is a technique of respiratory support that achieves extracorporeal decarboxylation at low blood flow (about 0.3–1.0 L/min) through an extracorporeal veno-venous or artero-venous circuit, without significant effect on blood oxygenation (25). Differently from O2, CO2 kinetics in blood is characterized by high solubility in plasma (1 L of blood contains around 500 mL of CO2), and steep linear dissociation curve without saturation in the physiologic range in which CO2 removal occurs (26,27). Therefore, extracorporeal blood flow rates as low as 500 mL/min would be theoretically required to eliminate the whole CO2 body production. However, several technical factors limit the efficiency of CO2 clearance by the artificial lung (27,28), which averages about 30% of total CO2 production at low blood flow (<1.0 L/min) (27).
ECCO2R technical properties
ECCO2R devices consist of a drainage cannula placed in a large central vein or artery, a membrane lung, and a return cannula into the venous system. In the membrane lung, a flow of gas containing little or no CO2, known as “sweep gas”, runs along one side of the membrane, ensuring the diffusion gradient from the blood on the other side (25,29).
Blood flow through ECCO2R circuits can be achieved in one of two ways. By exploiting high arterial pressure, pumpless artero-venous extracorporeal carbon dioxide removal (AV-ECCO2R) results in less blood trauma but requires large bore arterial cannulas and an adequate cardiac output. Veno-venous extracorporeal carbon dioxide removal (VV-ECCO2R) uses mechanical pumps, which draw blood from a large central vein and propel it into the same or another vein (25,29). Different configurations are available in modern ECCO2R systems, but the most common one is the dual lumen VV-ECCO2R (27). A single double-lumen cannula is placed in the right internal jugular or femoral vein. This configuration reduces the incidence of cannulation-associated adverse events and patient discomfort (28).
After the first experimental evidence of the feasibility of ECCO2R in the late 1970’s (30), improved understanding of the physiology of extracorporeal gas exchange and technological advances have led to the development of more sophisticated devices which provide CO2 removal with high efficiency and lower risk for adverse effects (26,28,29). Particularly, centrifugal or diagonal low-impact flow pumps, non-microporous polymethylpentene membrane lungs with complex fiber arrangement, and heparin-coating in the circuitry and membrane provide better biocompatibility and gas exchange efficiency and less plasma leak, allowing the use of smaller surface areas (0.67 to 3 m2) and a lower degree of anticoagulation (28,29). The newer ECCO2R devices are relatively simple to use since they require insertion of a small double-lumen cannula (up to 13–15 Fr) and work with very low blood flows (even in the range of 0.2–0.5 L/min). However, their performance in terms of CO2 removal remains limited, as they remove around one-third of the total metabolic CO2 production (typically 50–80 mL/min) (27).
Pathophysiological rationale for ECCO2R in COPD exacerbations
During COPD exacerbations the volume of CO2 eliminated via the lungs is reduced because of worsening of dynamic alveolar hyperinflation and ventilation-perfusion mismatching (26) with development of severe hypercapnia. In addition, in patients with COPD exacerbation CO2 production is estimated to be 23% higher than the normal value of 200–250 mL/min due to additional work of breathing and increased metabolism (26).
Therefore, during COPD exacerbation relieving the native lung from at least part of the CO2 elimination with ECCO2R could potentially improve the acid-base balance; reduce patient’s work of breathing with consequent reduction in respiratory rate and ventilator drive, and lower alveolar ventilation (27). Instead of mechanically increasing alveolar ventilation with IMV in the already hyperinflated lung to increase CO2 elimination, the application of ECCO2R may allow lower tidal volumes and respiratory rate, resulting in the extension of the expiratory time, suiting better the high expiratory time constant of the respiratory system with expiratory flow limitation. By these physiological mechanisms ECCO2R can counteract the vicious circle of dynamic hyperinflation and its detrimental respiratory and cardio-vascular consequences. The derived beneficial effects on respiratory mechanics, ventilatory muscle efficiency, work of breathing, and cardiovascular function may improve gas exchanges and relieve dyspnea, thus potentially preventing NIV failure or facilitate weaning from IMV (26,27) (Figure 1).
Physiological effects
The physiological benefits of the application of ECCO2R in patients with severe COPD exacerbations were highlighted in two recent systematic reviews (31,32). In a physiologic meta-analysis, the institution of ECCO2R was associated with a rapid and sustained improvement in pH, arterial partial pressure of carbon dioxide (PaCO2), and respiratory rate, but not in oxygenation (31). Moreover, improvement in pH continued between 1 and 24 h post cannulation, whereas PaCO2 and respiratory rate did not change after 1 h. This difference was hypothesized to be attributable to improvements in metabolic rather than respiratory acidosis (e.g., improved circulation, administration of diuretics or blood transfusions).
Moreover, the use of ECCO2R in patients with COPD exacerbation has been demonstrated to reduce inspiratory effort (33) and work of breathing (34), thus potentially contributing to the reduction in metabolic acidosis through a decrease in the intracellular acidosis within the respiratory muscles (34). These effects may also importantly result in a remarkable reduction of the total CO2 body production and hence of alveolar ventilation demand, creating a virtuous cycle (Figure 1).
Furthermore, treatment with ECCO2R may be beneficial by leading to an improvement in right ventricular function. Intermediate-high flow ECCO2R has proven to reduce right ventricular afterload and improve right ventricular function in COPD exacerbations (35). This effect was mainly ascribed to a reduction in hypoxic and hypercapnic pulmonary vasoconstriction, besides the decrease in airway pressures after the institution of ECCO2R. Given the known deleterious effect of acute (36) and chronic (37,38) pulmonary hypertension in patients with COPD exacerbation and other types of acute respiratory failure (39), this suggested hemodynamic effect of ECCO2R could be of clinical importance. However, further studies are needed to confirm these results and to broaden them to low-flow ECCO2R.
Clinical evidence
In the first anecdotal experience of ECCO2R applied in COPD exacerbation, a 19-year-old woman with bullous emphysema and lung infection failed various attempts of weaning from IMV (40). A CT scan showed the presence of hyperinflation of multiple lung bullae, likely maintained by the high tidal volumes required to provide adequate CO2 clearance. The application of a low blood flow (0.4–0.6 L/min) VV-ECCO2R device allowed the patient to be completely liberated from any artificial mean of respiratory support. Interestingly, a subsequent CT showed a decrease of the size of the bullae with expansion of the residual lung parenchyma, suggesting that the ECCO2R contributed to reduce the dynamic alveolar hyperinflation.
Since then, although high-quality studies are still lacking, several case reports and case series described the effective use of ECCO2R in patients with COPD exacerbations to avoid the initiation of IMV after NIV failure (41-51), and to facilitate early extubation in patients requiring IMV (50,52-54). Moreover, few case-control studies were published that assessed the effectiveness of ECCO2R in avoiding intubation in patients suffering from COPD exacerbations (55-57). A summary of the design, outcome, complication data, and device characteristics of these studies is reported in two recent systematic reviews (31,32). Following is a brief description of the studies characterized by the higher number of included patients.
Case series
A multicenter pilot study provided data in favor of the feasibility of dual lumen VV-ECCO2R for consistent removal of CO2 over several days at relatively low blood flows (43). Three groups of patients were studied: patients ventilated with NIV and with a very high likelihood of requiring IMV (n=7); patients who had failed two weaning attempts from NIV and refused IMV (n=2); patients who were already on IMV and had either failed two or more weaning attempts or failed one weaning attempt and did not wish to continue IMV (n=11). ECCO2R allowed to avoid IMV in all the patients in the first group and to wean off NIV both patients in the second group, whereas only 3/11 patients in the third group were weaned off IMV. One patient died as a result of a retroperitoneal bleed following catheterization. In their feasibility single-center study (53), Abrams et al. reported that all five patients included were successfully extubated within 24 h and ambulating within 48 h of ECCO2R support.
Spinelli et al. (45) showed in six patients with COPD exacerbation who received IMV after NIV failure that ECCO2R was functional in maintaining spontaneous breathing and in controlling patient’s respiratory rate. By progressively increasing the sweep gas flow, a respiratory rate of less than 15 breaths per minute could be achieved in all patients.
In a case series of 30 patients who refused endotracheal intubation after NIV failure (49), ECCO2R was associated with a significant decrease in mortality in comparison with 30 historical controls, who received conventional treatment with endotracheal intubation. None of the patients treated with ECCO2R experienced bleeding events; however, eight patients required the substitution of the circuit due to clotting.
In another small case series, Moss et al. (50) describes the use of VV-ECCO2R in 14 patients, including 5 patients with COPD exacerbation and 9 with ARDS. In total, four circuit-related complications were reported, none resulting in serious adverse outcomes. VV-ECCO2R allowed avoiding intubation or achieving early extubation in 4 out of 5 patients with COPD exacerbation.
In a recently published case series, Hilty et al. (51) described the efficiency and feasibility of VV-ECCO2R in two acute hypercapnic respiratory failure scenarios. Besides confirming the avoidance of IMV in 6 patients at risk of NIV failure (1 patient with COPD exacerbation), ECCO2R was effective in supporting the maintenance of lung protective ventilation, while correcting severe respiratory acidosis, in 14 patients supported with IMV (3 patients with COPD exacerbation).
Case control studies
In the first clinical study evaluating the effectiveness of ECCO2R to avoid intubation in patients with COPD exacerbation refractory to NIV, Kluge et al. (55) observed that the application of a mid-flow AV-ECCO2R device prevented intubation in 19/21 patients (90%), including 14 patients with COPD exacerbation. These patients were recruited in 4 hospitals and were retrospectively compared to a matched control group of 21 patients, who received IMV and were selected based on underlying diagnosis, age, simplified acute physiology score II (SAPS II) and pH at ICU admission. The AV-ECCO2R strategy was associated with significantly fewer tracheostomies (10% vs. 67%, P=0.004), and a trend toward shorter hospital length of stay (23 vs. 42 days, P=0.06). Considerable complications occurred, including two major and seven minor bleeding events, a femoral artery pseudoaneurysm and a case of heparin-induced thrombocytopenia type 2. Despite all efforts of matching, the patients supported with the AV-ECCO2R device had significantly worse hypercapnia before the initiation of the intervention than the controls. In addition, 43% of patients in this group were awaiting lung transplantation, but none in the control group.
In another multicenter retrospective study with historical control published by Del Sorbo et al. (56), a rigorous matching method (GenMatch) was applied to compare 25 patients supported with a low-flow VV-ECCO2R device because of the risk of NIV failure after COPD exacerbation with 21 patients who received only NIV. The addition of ECCO2R to NIV significantly decreased the risk of intubation (hazard ratio 0.27, 95% CI: 0.07–0.98). Among secondary endpoints, ICU and hospital length of stay were similar, whereas hospital mortality was significantly lower (8% vs. 33%, P=0.035) when ECCO2R was added. Thirteen patients (52%) experienced adverse events related to ECCO2R, mainly consisting of mechanical events (i.e., clots in the circuit, pump malfunction, membrane lung failure).
In their multicenter case-control study (57), Braune et al. investigated the feasibility and safety of a mid-flow VV-ECCO2R device to avoid IMV in 25 patients with COPD exacerbation after NIV failure. Intubation was avoided in 56% of patients; 7 patients were intubated because of progressive hypoxemia and 4 due to ventilatory failure despite ECCO2R and NIV. These patients were retrospectively compared with 25 patients selected by the following matching criteria: the presence of hypercapnic respiratory failure, age, SAPS II on ICU admission, and pH at the time of NIV failure. Although the duration of mechanical ventilation was significantly lower in the ECCO2R group (8 vs. 14 days, P=0.02), ICU and hospital length of stay did not differ significantly between the two groups, and neither did mortality. Fourteen major ECCO2R-associated adverse events were observed in 11 patients (44%).
Overall, despite its sound pathophysiologic rationale, current evidence regarding the use of ECCO2R in patients with COPD remains limited to low-quality studies with a non-randomized design, including important differences in patient selection, type of ECCO2R device used, and anticoagulation strategy (31). Moreover, length of stay and survival were not consistently influenced by ECCO2R when compared to controls (31,32). Finally, major complications related to ECCO2R therapy are frequent and may counterbalance the potential benefit of successful avoidance or early termination of intubation (58). More data will be forthcoming on the application of ECCO2R in the management of patients with COPD exacerbations from a number of ongoing or planned clinical trials (Table 1).

Full table
Complications
As previously described the application of ECCO2R in patients suffering from COPD exacerbations was associated with adverse events, which represent a major clinical concern because of their frequency and potential severity. These complications include patient-related events (i.e., worsening of hypoxemia; anticoagulation-related bleeding; hemolysis; heparin-induced thrombocytopenia), circuit placement events (i.e., cannulation site bleeding; cannulas malposition, displacement or kinking; vascular occlusion; thrombosis; hematoma or aneurism/pseudoaneurysm formation), and mechanical events (malfunctioning or failure of pump, membrane, gas exchanger; clots formation; air embolism) (27,31).
In the case control studies on the utilization of ECCO2R in COPD exacerbations, major complications ranged from 10–44% (55-57). More generally, in a systematic review that included 10 studies (86 patients), 47% experienced ECCO2R-related adverse events (31). Eleven major complications (i.e., complications causing death or life-threatening conditions in the absence of treatment, directly related to a device complication; transfusion ≥2 units of packed red cells; or the need for open surgery) and 30 minor complications (i.e., bleeding requiring <2 units of packed red cells; transient thrombocytopenia at most 90×109/L without clinical consequence; or non-life-threatening event related to catheter insertion) were reported. Of the 11 major complications, 73% were clinically significant bleeding episodes. Of the 30 minor complications, 43% were minor bleeding episodes related either to device insertion or systemic anticoagulation, 30% were related to device malfunction, and 13% consisted of transient thrombocytopenia.
Bleeding and thrombosis
The occurrence of bleeding events is the most frequent complications of ECCO2R. Indeed, the major advantage of the low flow is that relatively small vascular cannulas (13–17 Fr) can be used, although some double lumen cannulas can reach up to 24 Fr (57). However, the low flow may result in a high risk of clotting, thus requiring full anticoagulation (58). Major bleeding events occur in 10–36% patients (43,55-57), potentially resulting in life-threatening complications or death. Kalbhenn et al. recently assessed the blood cell injury and acquired coagulation disorders during ECCO2R support and reported ECCO2R to be associated with hemolysis, thrombocytopenia, acquired von Willebrand syndrome, and factor XIII deficiency, along with relevant clinical bleeding in 57% patients (59).
Nevertheless, many of the complications were caused by insufficient anticoagulation. Circuit clotting occurred in 24% of patients in the study from Del Sorbo et al. (56), whose device was set at very low flows (mean 255 mL/min). Of note, in 2 of these patients, the resulting severe respiratory acidosis necessitated endotracheal intubation. In the case series from Morelli et al. (49), circuit clotting was reported in 27% of patients and required the substitution of the circuit. Moreover, membrane lifetime was limited by obstructing clot formation in 61% of the membranes used in the case-series from Hilty et al. (51), who applied blood flows of 300–350 mL/min. Higher blood flows appear to be associated with less clotting events. Braune et al. (57) used mean blood flow of 1.3 L/min and reported thrombotic events in 8% patients, but more hemorrhagic complications (36% of major bleeding events).
These data highlight the challenging management of the delicate balance between hemorrhagic and thrombotic risk in these patients, and the uncertainty on the optimal therapeutic targets for anticoagulation during extracorporeal circulation (60). Furthermore, despite growing interest in more sophisticated point-of-care tests (e.g., rotational thromboelastometry and platelet aggregometry) for monitoring global coagulation function during ECCO2R, the evidence on the concordance between these techniques and conventional tests is still controversial (59,61,62) and further prospective studies are necessary to assess the usefulness of point-of-care coagulation monitoring methods in decreasing hemorrhagic and thromboembolic complications (63).
Other complications of ECCO2R
Among less frequent complications of ECCO2R support, major adverse events can be caused by vein and/or arterial cannulation, such as lower limb ischemia in AV-ECCO2R, which has been reported only in ARDS patients (64-66), femoral artery pseudoaneurysm (55,66), and vein perforation (43,56).
Along with the difficulty in predicting the clinical course of hypercapnic respiratory failure at an early stage (e.g., evolution of infiltrates, respiratory secretions, shock) (57), worsening of hypoxemia can occur during ECCO2R because pulmonary vasodilation resulting from excessive CO2 removal could abolish physiologic hypoxic vasoconstriction, thereby increasing pulmonary shunt fraction (67,68). In addition, excessive CO2 removal may lead to a reduction of tidal volume and increased risk of atelectasis, especially during spontaneous ventilation (69). Nonetheless, during severe COPD exacerbation with severe alveolar hyperinflation the occurrence of hypoxemia secondary to the use of ECCO2R is infrequent and can usually be corrected with modest doses of supplemental oxygen.
Future perspectives
Recent advances in technology are focused on the development of minimally invasive devices, which provide adequate CO2 removal with improved safety and ease of use, such as gas-exchange catheters (29). Moreover, innovative and promising methods to maximize CO2 removal, such as respiratory dialysis that uses bicarbonate-free dialysates, are being investigated in animal models (29). However, these methods may be impractical for clinical use due to acid-base derangements, hemolysis, cardiac arrhythmias, and depletion of micronutrients, despite several approaches to replace bicarbonate have been attempted (29,32). Other techniques that have been evaluated include the combination of ECCO2R and continuous renal replacement therapies, acidification of the blood with lactic acid, the addition of carbonic anhydrase to the membrane, and electrodialysis (32,70). These strategies may improve physiologic advantages of ECCO2R, while at the same time reducing its risks; however studies demonstrating safety and efficacy are needed before implementing these technological innovations into clinical practice.
Conclusions
The available literature does not allow drawing any conclusion on the risk-benefit balance of ECCO2R in COPD exacerbations and most authors advocate the urgent need for high-quality, randomized, controlled trials assessing the effect of ECCO2R on relevant clinical outcomes (31,32,56,58,71). However, the choice of the appropriate investigational strategy can present considerable challenges. A study designed to determine whether ECCO2R improves outcome by accelerating weaning from IMV would expose patients to the risk of complications from two simultaneously applied invasive interventions, ECCO2R and IMV. A study designed to determine whether ECCO2R improves outcome by preventing IMV in NIV failure would carry the risk of exposing to ECCO2R complications patients that would not require IMV. Overall, future trials will need to address the question whether the risks associated with IMV outweigh the ECCO2R-related complications in patients with severe COPD exacerbations. While waiting for more rigorous evidence from randomized trials, ECCO2R for patients with COPD exacerbations should be considered only as an experimental technique and applied within a research context in experienced centers (25,31,32).
Despite the impressive technological advances and fascinating future perspectives, the hopeful report from Kolobow et al. in 1977 retains its relevance in the present (30): “We believe that carbon dioxide removal through the carbon dioxide membrane lung may be useful in management of ventilatory problems, prime among them being uncontrollable bronchopleural fistula, and those conditions where use of an artificial ventilator is not desired or is contraindicated, or where it must be terminated”.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Global Strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease (GOLD) 2017. Available online: http://goldcopd.org
- Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 2017;389:1931-40. [Crossref] [PubMed]
- Khakban A, Sin DD, Fitzgerald JM, et al. The Projected Epidemic of Chronic Obstructive Pulmonary Disease Hospitalizations over the Next 15 Years. A Population-based Perspective. Am J Respir Crit Care Med 2017;195:287-91. [PubMed]
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med 2017;5:691-706. [Crossref] [PubMed]
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- Donaldson GC, Wedzicha JA. COPD exacerbations. 1: Epidemiology. Thorax 2006;61:164-8. [Crossref] [PubMed]
- Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, et al. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J 2011;37:508-15. [Crossref] [PubMed]
- Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med 2014;35:157-63. [Crossref] [PubMed]
- Laghi F, Goyal A. Auto-PEEP in respiratory failure. Minerva Anestesiol 2012;78:201-21. [PubMed]
- O'Donnell DE, Parker CM. COPD exacerbations. 3 Pathophysiology. Thorax 2006;61:354-61. [Crossref] [PubMed]
- Marini JJ. Dynamic hyperinflation and auto-positive end-expiratory pressure: lessons learned over 30 years. Am J Respir Crit Care Med 2011;184:756-62. [Crossref] [PubMed]
- Barberà JA, Roca J, Ferrer A, et al. Mechanisms of worsening gas exchange during acute exacerbations of chronic obstructive pulmonary disease. Eur Respir J 1997;10:1285-91. [Crossref] [PubMed]
- Loring SH, Garcia-jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol 2009;107:309-14. [Crossref] [PubMed]
- Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med 2012;185:152-9. [Crossref] [PubMed]
- Bott J, Carroll MP, Conway JH, et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet 1993;341:1555-7. [Crossref] [PubMed]
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995;333:817-22. [Crossref] [PubMed]
- Kramer N, Meyer TJ, Meharg J, et al. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 1995;151:1799-806. [Crossref] [PubMed]
- Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet 2000;355:1931-5. [Crossref] [PubMed]
- Conti G, Antonelli M, Navalesi P, et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med 2002;28:1701-7. [Crossref] [PubMed]
- Squadrone E, Frigerio P, Fogliati C, et al. Noninvasive vs invasive ventilation in COPD patients with severe acute respiratory failure deemed to require ventilatory assistance. Intensive Care Med 2004;30:1303-10. [Crossref] [PubMed]
- Lindenauer PK, Stefan MS, Shieh MS, et al. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med 2014;174:1982-93. [Crossref] [PubMed]
- Stefan MS, Nathanson BH, Higgins TL, et al. Comparative Effectiveness of Noninvasive and Invasive Ventilation in Critically Ill Patients With Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Crit Care Med 2015;43:1386-94. [Crossref] [PubMed]
- Carlucci A, Richard JC, Wysocki M, et al. Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med 2001;163:874-80. [Crossref] [PubMed]
- Carrillo A, Ferrer M, Gonzalez-diaz G, et al. Noninvasive ventilation in acute hypercapnic respiratory failure caused by obesity hypoventilation syndrome and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:1279-85. [Crossref] [PubMed]
- Camporota L, Barrett N. Current Applications for the Use of Extracorporeal Carbon Dioxide Removal in Critically Ill Patients. Biomed Res Int 2016;2016:9781695. [PubMed]
- Lund LW, Federspiel WJ. Removing extra CO2 in COPD patients. Curr Respir Care Rep 2013;2:131-8. [Crossref] [PubMed]
- Morelli A, Del Sorbo L, Pesenti A, et al. Extracorporeal carbon dioxide removal (ECCO2R) in patients with acute respiratory failure. Intensive Care Med 2017;43:519-30. [Crossref] [PubMed]
- Morimont P, Batchinsky A, Lambermont B. Update on the role of extracorporeal CO? removal as an adjunct to mechanical ventilation in ARDS. Crit Care 2015;19:117. [Crossref] [PubMed]
- Cove ME, Maclaren G, Federspiel WJ, et al. Bench to bedside review: Extracorporeal carbon dioxide removal, past present and future. Crit Care 2012;16:232. [Crossref] [PubMed]
- Kolobow T, Gattinoni L, Tomlinson TA, et al. Control of breathing using an extracorporeal membrane lung. Anesthesiology 1977;46:138-41. [Crossref] [PubMed]
- Sklar MC, Beloncle F, Katsios CM, et al. Extracorporeal carbon dioxide removal in patients with chronic obstructive pul- monary disease: a systematic review. Intensive Care Med 2015;41:1752-62. [Crossref] [PubMed]
- Taccone FS, Malfertheiner MV, Ferrari F, et al. Extracorporeal CO2 removal in critically ill patients: a systematic review. Minerva Anestesiol 2017;83:762-72. [PubMed]
- Pisani L, Fasano L, Corcione N, et al. Effects of extracorporeal CO2 removal on inspiratory effort and respiratory pattern in patients who fail weaning from mechanical ventilation. Am J Respir Crit Care Med 2015;192:1392-4. [Crossref] [PubMed]
- Diehl JL, Piquilloud L, Richard JC, et al. Effects of extracorporeal carbon dioxide removal on work of breathing in patients with chronic obstructive pulmonary disease. Intensive Care Med 2016;42:951-2. [Crossref] [PubMed]
- Karagiannidis C, Strassmann S, Philipp A, et al. Veno-venous extracorporeal CO2 removal improves pulmonary hypertension in acute exacerbation of severe COPD. Intensive Care Med 2015;41:1509-10. [Crossref] [PubMed]
- Wells JM, Morrison JB, Bhatt SP, et al. Pulmonary artery enlargement is associated with cardiac injury during severe exacerbations of COPD. Chest 2016;149:1197-204. [Crossref] [PubMed]
- McGhan R, Radcliff T, Fish R, et al. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest 2007;132:1748-55. [Crossref] [PubMed]
- Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012;367:913-21. [Crossref] [PubMed]
- Zochios V, Parhar K, Tunnicliffe W, et al. The right ventricle in ARDS. Chest 2017;152:181-93. [Crossref] [PubMed]
- Pesenti A, Rossi GP, Pelosi P, et al. Percutaneous extracorporeal CO2 removal in a patient with bullous emphysema with recurrent bilateral pneumothoraces and respiratory failure. Anesthesiology 1990;72:571-3. [Crossref] [PubMed]
- Crotti S, Lissoni A, Tubiolo D, et al. Artificial lung as an alternative to mechanical ventilation in COPD exacerbation. Eur Respir J 2012;39:212-5. [Crossref] [PubMed]
- Brederlau J, Wurmb T, Wilczek S, et al. Extracorporeal lung assist might avoid invasive ventilation in exacerbation of COPD. Eur Respir J 2012;40:783-5. [Crossref] [PubMed]
- Burki NK, Mani RK, Herth FJ, et al. A novel extracorporeal CO2 removal system: Results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest 2013;143:678-86. [Crossref] [PubMed]
- Bonin F, Sommerwerck U, Lund LW, et al. Avoidance of intubation during acute exacerbation of chronic obstructive pulmonary disease for a lung transplant candidate using extracorporeal carbon dioxide removal with the Hemolung. J Thorac Cardiovasc Surg 2013;145:e43-4. [Crossref] [PubMed]
- Spinelli E, Crotti S, Zacchetti L, et al. Effect of extracorporeal CO2 removal on respiratory rate in spontaneously breathing patients with chronic obstructive pulmonary disease exacerbation. Crit Care 2013;17:128. [Crossref] [PubMed]
- Mani RK, Schmidt W, Lund LW, et al. Respiratory dialysis for avoidance of intubation in acute exacerbation of COPD. ASAIO J 2013;59:675-8. [Crossref] [PubMed]
- Cole S, Barrett N, Glover G, et al. Extracorporeal carbon dioxide removal as an alternative to endotracheal intubation for non-invasive ventilation failure in acute exacerbation of COPD. JICS 2014;15:344-6.
- Hermann A, Staudinger T, Bojic A, et al. First experience with a new miniaturized pump-driven venovenous extracorporeal CO2 removal system (iLA Activve): a retrospective data analysis. ASAIO J 2014;60:342-7. [Crossref] [PubMed]
- Morelli A, D’Egidio A, Orecchioni A, et al. Extracorporeal CO2 removal in hypercapnic patients who fail noninvasive ventilation and refuse endotracheal intubation: a case series. Intensive Care Med Exp 2015;3:A824. [Crossref]
- Moss CE, Galtrey EJ, Camporota L, et al. A retrospective observational case series of low-flow venovenous extracorporeal carbon dioxide removal use in patients with respiratory failure. ASAIO J 2016;62:458-62. [Crossref] [PubMed]
- Hilty MP, Riva T, Cottini SR, et al. Low flw veno-venous extra-corporeal CO2 removal for acute hypercapnic respiratory failure. Minerva Anestesiol 2017;83:812-23. [PubMed]
- Cardenas VJ Jr, Lynch JE, Ates R, et al. Venovenous carbon dioxide removal in chronic obstructive pulmonary disease: experience in one patient. ASAIO J 2009;55:420-2. [Crossref] [PubMed]
- Abrams DC, Brenner K, Burkart KM, et al. Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013;10:307-14. [Crossref] [PubMed]
- Roncon-Albuquerque R, Carona G, Neves A, et al. Venovenous extracorporeal CO2 removal for early extubation in COPD exacerbations requiring invasive mechanical ventilation. Intensive Care Med 2014;40:1969-70. [Crossref] [PubMed]
- Kluge S, Braune SA, Engel M, et al. Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med 2012;38:1632-9. [Crossref] [PubMed]
- Del Sorbo L, Pisani L, Filippini C, et al. Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med 2015;43:120-7. [Crossref] [PubMed]
- Braune S, Sieweke A, Brettner F, et al. The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case–control study. Intensive Care Med 2016;42:1437-44. [Crossref] [PubMed]
- Beloncle F, Brochard L. Extracorporeal CO2 removal for chronic obstructive pulmonary disease: too risky or ready for a trial? Crit Care Med 2015;43:245-6. [Crossref] [PubMed]
- Kalbhenn J, Neuffer N, Zieger B, et al. Is extracorporeal CO2 removal really "safe" and "less" invasive? Observation of blood injury and coagulation impairment during ECCO2R. ASAIO J 2017;63:666-71. [Crossref] [PubMed]
- Sklar MC, Sy E, Lequier L, et al. Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure: a systematic review. Ann Am Thorac Soc 2016;13:2242-50. [Crossref] [PubMed]
- Nair P, Hoechter DJ, Buscher H, et al. Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J Cardiothorac Vasc Anesth 2015;29:288-96. [Crossref] [PubMed]
- Prakash S, Wiersema UF, Bihari S, et al. Discordance between ROTEM? clotting time and conventional tests during unfractionated heparin-based anticoagulation in intensive care patients on extracorporeal membrane oxygenation. Anaesth Intensive Care 2016;44:85-92. [PubMed]
- Bolliger D, Zenklusen U, Tanaka KA. Point-of-care coagulation management algorithms during ECMO support: are we there yet? Minerva Anestesiol 2016;82:1000-9. [PubMed]
- Bein T, Weber F, Philipp A, et al. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med 2006;34:1372-7. [Crossref] [PubMed]
- Zimmermann M, Bein T, Arlt M, et al. Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: a prospective pilot study. Crit Care 2009;13:R10. [Crossref] [PubMed]
- Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus “conventional” protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med 2013;39:847-56. [Crossref] [PubMed]
- Hermann A, Riss K, Schellongowski P, et al. A novel pump-driven veno-venous gas exchange system during extracorporeal CO2-removal. Intensive Care Med 2015;41:1773-80. [Crossref] [PubMed]
- Crotti S, Bottino N, Ruggeri GM, et al. Spontaneous breathing during extracorporeal membrane oxygenation in acute respiratory failure. Anesthesiology 2017;126:678-87. [Crossref] [PubMed]
- Del Sorbo L, Fan E, Nava S, et al. ECCO2R in COPD exacerbation only for the right patients and with the right strategy. Intensive Care Med 2016;42:1830-1. [Crossref] [PubMed]
- Zanella A, Castagna L, Salerno D, et al. Respiratory electrodialysis. A novel, highly efficient extracorporeal CO2 removal technique. Am J Respir Crit Care Med 2015;192:719-26. [Crossref] [PubMed]
- Grasselli G, Pesenti A. Extracorporeal CO2 removal: a powerful tool to be handled with care. Minerva Anestesiol 2017;83:682-4. [PubMed]


