National use of total hip arthroplasty among patients with a history of breast, lung, prostate, colon or bladder cancer—an analysis of the Medicare population
Introduction
Total hip arthroplasty (THA), is a commonly performed procedure in the United States. Previous studies have estimated that around 600,000 THA procedures are done each year (1). THA provides substantial quality of life, allows return to work, and is commonly employed following fractures (2). It has been proven to improve patient outcomes and to be cost effective (2,3). Recent data has demonstrated that patients with cancer are at increased risk of complications and thus this aspect of arthroplasty has gained more attention (4-6).
A recent annual report from the American National Cancer Institute (NCI), demonstrates that overall, cancer incidence continues to have wide variation but also shows that mortality has decreased in recent years, demonstrating that more of the patients with this condition are living in remission (7). Recent changes in treatment to these cancers, such as stereotactic radiation, systemic targeted treatment, hormonal receptor targeting molecules and novel chemotherapy may have caused an increase in life expectancy and a change in the use of arthroplasty in these patients (8-10). Understanding the utilization of THA among patients diagnosed with a solid organ malignancy is crucial as previous studies have demonstrated worse outcomes in this patient population but not its current national use (11-14).
The purpose of this study was to perform an epidemiological analysis of the use of THA among patients with a diagnosis of a solid organ malignancy.
Methods
We conducted a retrospective epidemiological review of the entire Medicare files through the use of the Pearldiver Supercomputer (Warsaw, IN, USA). This technology allows the study of the entire Medicare records from 2005 to 2012 by coding with Current Procedural Terminology (CPT) codes and International Classification of Disease (ICD) ninth revision codes. The five most common malignancies according to the National Cancer were selected for analysis. The selected ones were based on the NCI of the United States, which provides an annual report on cancer based on data from the American Cancer Society (ACS), the Centers for Disease Control (CDC), the NCI and the North American Association of Central Cancer Registries (NAACCR) (7). This data is combined and an annual report is made available to the public. The most recent report is based on data from 1975 to 2012 and describes the top five most common malignancies to be: breast, lung, prostate, colon and bladder cancer, and thus were selected for this study. The ICD code for THA 81.51 was used to determine which patients received an operation. Yearly incidence and growth were studied. Descriptive statistics as well as linear regressions were used to analyze trends over time. An alpha value of less than or equal to 0.05 was deemed as significant. Statistical analysis was performed with SPSS version 20 (IBM, Armonk, NY, USA).
Results
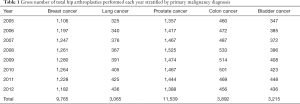
Between 2005 and 2012, 14,145,558 patients were diagnosed with one of the five types of cancer. Results have been separated according to type of malignancy (Table 1). The prevalence of THA among our entire patient population was 0.29%.
Full table
Breast cancer
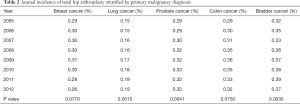
There were a total of 3,326,008 patients with a primary diagnosis of breast cancer. The yearly number of patients did not change significantly throughout the study period (P=0.97). Of these patients, only 9,765 (0.29%) underwent THA throughout the study period, with an average of 1,221 (SD 57.3) procedures done each year. There was no significant change in number of procedures done each year among patients with breast cancer diagnosis (P=0.173). The incidence of THA among patients with a diagnosis of breast cancer ranged from 0.26% annually to 0.31% with a mean of 0.29%, which did not change significantly over time (P=0.077) (Table 2). The mean incidence of THA among breast cancer patients was 294 THAs per 100,000 patients SD 14.9.
Full table
Lung cancer
Our search identified a total of 1,817,651 patients with a primary diagnosis of lung cancer. The gross number of patients increased significantly over time (P=0.019), as did the number of THAs, which averaged 383 (SD 39) per year (P<0.001). Of these patients, there were 3,605 (0.17%) who received a THA. The mean incidence of THAs in lung cancer patients was 0.17% with a range from 0.15% to 0.19%. This annual incidence increased significantly over time (P=0.001) (Table 2). There was a mean incidence of 168 (SD 15) THAs per 100,000 patients.
Prostate cancer
There were a total of 3,699,433 patients diagnosed with prostate cancer with a significant decrease in total patients with this diagnosis over the 8-year study period (P=0.0036). There were 11,539 (0.31%) prostate cancer patients who received a THA. The annual number of THA amongst this population did not change significantly (P=0.349), mean 1442 (SD 53). The annual incidence of THA in prostate cancer patients increased significantly from 0.29% in 2005 to 0.32% in 2012 (P=0.0041) (Table 2) with a mean of 0.31%. The mean incidence was 312 (SD 16) THAs per 100,000 patients.
Colon cancer
We identified a total of 1,189,417 patients with colon cancer. The number of patients with this diagnosis decreased significantly over time (P<0.001). Of these patients, only 3,892 (0.33%) received a THA. The mean number of procedures done annually was 486 (SD 27). The annual incidence ranged from 0.29% to 0.36% over the 8-year study period with a 0.33% mean incidence, which did not change significantly (P=0.075) (Table 2) throughout the study period. The mean incidence was 328 (SD 25) THAs per 100,000 patients.
Bladder cancer
There were a total of 893,755 patients with a primary diagnosis of bladder cancer. The number these patients increased significantly over time (P=0.028). Only 3,215 (0.36%) patients with this primary diagnosis underwent THA. The mean annual number of procedures was 401 (SD 33), range 347 to 448. The number of procedures increased significantly over time (P<0.001). There was a significant increase in the annual incidence of THAs, with a mean incidence of 0.36% with a range of 0.32% to 0.39% during the study period (P=0.003) (Table 2). The mean incidence was 359 (SD 26) THAs per 100,000 patients.
Discussion
Cancer is a devastating disease, and is the second most common cause of mortality in the USA. Recently there have been reports that there are over 14.5 million cancer survivors (CS), which is a number that is expected to rise to nearly 20 million patients by 2024 (15-18). These figures are alarming and demonstrate the success of oncologic treatment, improved screening methods and an aging population among other factors.
With many of these CS achieving significant improvements in quality of life and return to functional activity, the onset of osteoarthritis may continue to present in this subset of patients. Degenerative arthritis combined with systemic inflammation during and possibly after cancer, may play an important role in joint degeneration leading to further requirement of care for such a problem.
Concomitantly with the increase in CS, recent literature has demonstrated that THA is a procedure that is performed over 300,000 times annually and growing significantly (19,20).
Advances in oncologic treatment modalities have led to increased survival of cancer patients (21,22). These patients may require unique preoperative measures to ensure good clinical outcomes. Thus it is necessary to understand the current trends in CS patients undergoing THA. The primary findings of our study demonstrate that THA is performed in less than 0.5% of patients with a primary malignancy diagnosis compared with the normal population which has been recently reported to be around 5% in octogenarians and over 0.83% for the entire US population, even though the number of CS has recently been described as on the rise (22,23).
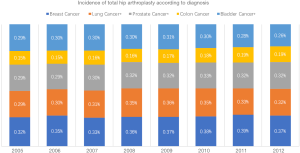
Our results also show that although the gross number of THA in this population is low, there has been an increased incidence in patients with lung, prostate and bladder cancer, while the incidence of this procedure in breast and colon cancer patients has remained constant (Figure 1). Various reasons may have caused these changes. Patients with malignancy have increased risk of avascular necrosis of the femoral head due to chemotherapy, dexamethasone therapy and vascular injury, creating a hypercoagulability state that may lead to disturbance of the circulation to the femoral head (24,25). This unfortunate state may even be occurring for a prolonged period of time it the diagnosis of malignancy was made at an early age (26).
Contrary to the constant rate of THA in breast cancer patients found in our study, a retrospective Norwegian registry study of 8,563 THA with a preoperative diagnosed malignancy found an increased THA incidence in breast cancer patients [SIR =1.13 (95% CI, 1.08–1.18)] (27). This is thought to be due to the effects of chemotherapy and aromatase inhibitors increasing the risk of osteoporosis (28,29). However, these studies included a substantially younger age range than our Medicare patient population. Current literature has shown that the incidence rate increases by approximately 8–9% per year during the premenopausal years while slowing to approximately 2–3% per year after menopause (30,31). This may explain the current study findings.
Our data demonstrate that more patients with oncologic diagnosis are undergoing THA, and is expected that these numbers would increase over the following years, which will certainly allow more patients to undergo THA if required, but is important to know that this particular population as previously reported has increased odds of worse outcomes (4,13) such as deep vein thrombosis, pulmonary embolism which has led to certain groups to suggest more aggressive thromboprophylaxis and patient selection in these population (4,14).
Studies have failed to identify the role of malignancy on periprosthetic joint infections (PJI) following THA. A large retrospective study by Pulido et al., analyzed different factors such as patient characteristics (race, comorbidities, ASA classification, body mass index, anemia, etc.), surgical factors and postoperative factors in order to determine the influence of several of these on the development of infection. Although malignancy was not determined to be one of those precipitating factors, anemia, higher creatinine, and longer hospital stay were all found to increase significantly the chances of PJI, which are factors often affecting oncologic patients/CS (11). Contrary to this, Bozic et al. found that metastatic cancer increased the adjusted 90-day hazard ratio (HR) for mortality (HR =3.14) among patients who underwent THA thus demonstrating the value of further analyzing and being vigilant with patients with cancer. This same group of authors also identified metastatic disease as a marker for 90-day TKA morbidity (AHR =4.4) and more significantly also demonstrated that metastatic disease increases the rate of PJI significantly (AHR =1.59) (14,32).
Patients with active or recent oncologic pathology also require special attention. With the limited evidence available of long-term outcomes, it is often difficult to advise these patients on risks specific or increased in that patient population. We hypothesize that with the creation of multidisciplinary groups that include an oncology team, hospitalists, physical therapists and orthopedic surgeons, patients can be optimized before surgery and care plans can be offered in regards to medication use, arthroplasty bearings, post-operative physical therapy and care. We hypothesize that such venture can decrease complications as deep venous thrombosis, pulmonary embolisms and wound discharge or infection. Similarly, a multidisciplinary approach is useful, as prognosis and life expectancy would be factored into the discussion, given that many of these patients, especially the ones with metastatic disease, may have a limited prognosis (33).
Surgical technique is also of great importance. A recent study led by Parvizi et al. demonstrated that patients with a recent history of pelvic radiation for prostate cancer do not have an increased risk of failure after uncemented THA, as the integration of the acetabular component to the pelvis is not compromised. In contrast to this, older studies involving patients with colorectal cancer, gynecological malignancies and lymphoma have identified greater failure of THA in patients who undergo radiation therapy. Thus further evidence is still required to conclude the better technique for this subset of patients (34).
Limitations
Our study is not without limitations. Our retrospective analysis of the entire Medicare data might have incurred in retrospective bias, thus only allowing this study to be classified as a level of evidence IV study. Another weakness to our study is that we did not account for the indication of the THA nor did we study the complication rates amongst this population. Although these limitations exist, we believe that there is a need for an epidemiological study that evaluates the use of this procedure on a patient population that will continue to grow in the upcoming years according to National statistics, which will allow healthcare policy to include this growing patient population.
Conclusions
THA is not a commonly performed procedure among patients with a primary solid malignancy, with it being performed on less than 1% of patients with these diagnoses. In the recent years there has been an increased use of THA among patients with lung, prostate and bladder cancer, which may be indicative of greater cancer survivorship and better THA efficacy. The use of this procedure on breast and colon cancer patients has remained constant in the past years.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780-5. [PubMed]
- Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA 1996;275:858-65. [Crossref] [PubMed]
- Nichols CI, Vose JG. Clinical Outcomes and Costs Within 90 Days of Primary or Revision Total Joint Arthroplasty. J Arthroplasty 2016;31:1400-6.e3. [Crossref] [PubMed]
- Karam JA, Huang RC, Abraham JA, et al. Total joint arthroplasty in cancer patients. J Arthroplasty 2015;30:758-61. [Crossref] [PubMed]
- Houdek MT, Wagner ER, Wilke BK, et al. Late complications and long-term outcomes following aseptic revision of a hip arthroplasty performed for oncological resection. Hip Int 2015;25:428-34. [Crossref] [PubMed]
- Thambapillary S, Dimitriou R, Makridis KG, et al. Implant longevity, complications and functional outcome following proximal femoral arthroplasty for musculoskeletal tumors: a systematic review. J Arthroplasty 2013;28:1381-5. [Crossref] [PubMed]
- Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016;122:1312-37. [Crossref] [PubMed]
- Harlos C, Musto G, Lambert P, et al. Androgen pathway manipulation and survival in patients with lung cancer. Horm Cancer 2015;6:120-7. [Crossref] [PubMed]
- Ennis RD, Peschel RE. Radiation therapy for prostate cancer. Long-term results and implications for future advances. Cancer 1993;72:2644-50. [Crossref] [PubMed]
- Nakano M, Fujisue M, Tashima R, et al. Survival time according to the year of recurrence and subtype in recurrent breast cancer. Breast 2015;24:588-93. [Crossref] [PubMed]
- Pulido L, Ghanem E, Joshi A, et al. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 2008;466:1710-5. [Crossref] [PubMed]
- Memtsoudis SG, Sun X, Chiu YL, et al. Utilization of critical care services among patients undergoing total hip and knee arthroplasty: epidemiology and risk factors. Anesthesiology 2012;117:107-16. [Crossref] [PubMed]
- Mantilla CB, Wass CT, Goodrich KA, et al. Risk for perioperative myocardial infarction and mortality in patients undergoing hip or knee arthroplasty: the role of anemia. Transfusion 2011;51:82-91. [Crossref] [PubMed]
- Bozic KJ, Lau E, Kurtz S, et al. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am 2012;94:794-800. [Crossref] [PubMed]
- Kale HP, Carroll NV. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer 2016;122:283-9. [Crossref] [PubMed]
- Heins MJ, Korevaar JC, Hopman PE, et al. Health-related quality of life and health care use in cancer survivors compared with patients with chronic diseases. Cancer 2016;122:962-70. [Crossref] [PubMed]
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784-92. [Crossref] [PubMed]
- Elston Lafata J, Simpkins J, Schultz L, et al. Routine surveillance care after cancer treatment with curative intent. Med Care 2005;43:592-9. [Crossref] [PubMed]
- Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res 2008;466:914-9. [Crossref] [PubMed]
- Kurtz S, Mowat F, Ong K, et al. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am 2005;87:1487-97. [PubMed]
- Cho H, Mariotto AB, Schwartz LM, et al. When do changes in cancer survival mean progress? The insight from population incidence and mortality. J Natl Cancer Inst Monogr 2014;2014:187-97. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of Total Hip and Knee Replacement in the United States. J Bone Joint Surg Am 2015;97:1386-97. [Crossref] [PubMed]
- Gogas H, Fennelly D. Avascular necrosis following extensive chemotherapy and dexamethasone treatment in a patient with advanced ovarian cancer: case report and review of the literature. Gynecol Oncol 1996;63:379-81. [Crossref] [PubMed]
- Lykissas MG, Gelalis ID, Kostas-Agnantis IP, et al. The role of hypercoagulability in the development of osteonecrosis of the femoral head. Orthop Rev (Pavia) 2012;4:e17. [Crossref] [PubMed]
- Salem KH, Brockert AK, Mertens R, et al. Avascular necrosis after chemotherapy for haematological malignancy in childhood. Bone Joint J 2013;95-B:1708-13. [Crossref] [PubMed]
- Dybvik E, Furnes O, Fosså SD, et al. Long-term risk of receiving a total hip replacement in cancer patients. Cancer Epidemiol 2009;33:235-41. [Crossref] [PubMed]
- Yamamoto Y, Iwase H. Safety profiles of aromatase inhibitors and selective estrogen-receptor modulators in the treatment of early breast cancer. Int J Clin Oncol 2008;13:384-94. [Crossref] [PubMed]
- Molina JR, Barton DL, Loprinzi CL. Chemotherapy-induced ovarian failure: manifestations and management. Drug Saf 2005;28:401-16. [Crossref] [PubMed]
- Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses' Health Study. Am J Epidemiol 2000;152:950-64. [Crossref] [PubMed]
- Robert A. Smith LAB, Joan Kramer, et al. Epidemiology of Breast Cancer. In: Bassett L. editor. Breast Imaging. Philadelphia: Saunders, 2011:25-55.
- Bozic KJ, Lau E, Kurtz S, et al. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin Orthop Relat Res 2012;470:130-7. [Crossref] [PubMed]
- Schneiderbauer MM, von Knoch M, Schleck CD, et al. Patient survival after hip arthroplasty for metastatic disease of the hip. J Bone Joint Surg Am 2004;86-A:1684-9. [Crossref] [PubMed]
- Kim KI, Klein GR, Sleeper J, et al. Uncemented total hip arthroplasty in patients with a history of pelvic irradiation for prostate cancer. J Bone Joint Surg Am 2007;89:798-805. [PubMed]


