New trends in antitumor vaccines in melanoma
Immunology basics for designing antitumor vaccines
The antitumor immune response can be divided into two key steps: priming and effector phases.
In the priming phase, tumor antigens released by dying tumor cells are captured by dendritic cells, the professional antigen-presenting cells. In the steady state, antigen capture in the absence of danger signals leads to immune ignorance or tolerance. Immunogenic tumor cell death gives rise to the release of danger signals recognized by the molecular pattern receptors in the dendritic cells. The capture of tumor antigen while the dendritic cells sense the danger signals promotes the maturation of the dendritic cell (1). These mature dendritic cells migrate to the draining lymph node and are able to provide three signals to the T lymphocytes. Signal 1 is provided by the major histocompatibility (MHC) molecules that will display the tumor antigen to the T cell receptor (TCR) of the T lymphocytes. Signal 2 is provided by positive and negative costimulatory molecules. This second signal modulates the type of immune response to adapt the effector immune response to the danger sensed by the dendritic cells in the damaged tissue. Finally, signal 3 consists of the cytokines released in the immune synapse formed by the dendritic cells and the T lymphocytes. These provide an additional signal to shape the type of immune response (2).
As a result of this priming phase, an effector immune response is generated. The ideal antitumor immune response is composed of a coordinated network of cells of the innate and adaptative immune responses that exert cytotoxic mechanisms to kill tumor cells (3). The recent clinical success of monoclonal antibodies that target the PD-1/PD-L1 pathway demonstrates that in a fraction of tumor patients an endogenous antitumor immune response has been developed. In these patients, the mechanisms of adaptive resistance limit the effector immune response and such patients can benefit from a treatment that tips the balance in favor of the antitumor immune response (4).
However, in a large percentage of patients, there is a defect in the priming phase that prevents the development of an endogenous immune response. Therapeutic vaccines can overcome this hurdle.
Vaccines are composed of an antigen that provides signal 1 and an adjuvant to provide signals 2 and 3. Inactivated tumor cells or tumor cell lysates can be used to design antigen-unspecific vaccines. To introduce specific tumor-associated antigens into the vaccine, protein or peptides derived from selected tumor antigens must be produced and purified. Alternatively, these antigenic proteins or peptides can be expressed in the host using non-viral or viral gene therapy strategies. The antigens can be injected formulated with the adjuvant or can be loaded ex vivo into antigen presenting cells isolated from the patients.
Classic adjuvants are composed of mineral salts such as alum or oil emulsions. Novel adjuvants are being developed using molecules targeting specific molecular pattern receptors such as toll-like receptors or pro-inflammatory cytokines such as GM-CSF or IL-12 (5-7). CpG, a ligand of TLR-9 and poly I:C, a TLR-3 ligand, are among the most potent TLR ligands in cancer immunotherapy (8,9). Interestingly, synergistic combinations of different TLRs have been found and novel molecular pattern receptors such as the STING pathway widen the druggable pathways (10). Another strategy to activate the molecular pattern receptors is the use of viral and non-viral gene therapy vectors. These vectors are able to induce the release of tumor antigens in the context of immunogenic cell death. Gene therapy vectors encoding pro-inflammatory cytokines are able to induce an immune response against several tumoral antigens, avoiding the need for tumor-associated antigen identification (5,6,11,12). Finally, the combination of antigen delivery and antigen presenting cell maturation can be achieved with protein vectors that target in vivo dendritic cells (6,9).
We have demonstrated that the eradication of large tumors in mice can be achieved with a combined treatment. The treatment must include a drug to provide danger signals to the innate immune system. In our hands, the best drugs are polyI:C, a TLR3 ligand, and CpGs, a TLR9 ligand. Secondly, the treatment must include a vaccine. We have demonstrated potent antitumor efficacy with vaccines based on a detoxified version of the adenylate cyclase of Bordetella pertussis and with a fragment of the extracellular domain of fibronectin. The third component of a vaccine must keep in check the main regulatory immune system that blocks the antitumor activity of immune effector cells. In mouse models, a single low dose of cyclophosphamide is extremely potent due to the combination of T regulatory cell elimination and remodeling of the tumor-associated myeloid cells (9,13).
Lessons from clinical trials in melanoma
In 2004, Rosenberg et al. published a review of all the clinical trials that they performed at NHI to test antitumor vaccines in melanoma. The clinical experience included different adjuvants and delivery vehicles such as viruses, proteins, and peptides. The antigens assayed covered a broad spectrum of tumor-associated antigens. Their objective response rate was as low as 2.6% (14).
Since then, several anti-melanoma vaccines have been tested in phase III clinical trials for the treatment of advanced melanoma. However, none have been commercialized.
The gp100 peptide was formulated with Montanide ISA-51 and combined with systemic interleukin-2. This vaccine was administered to 185 patients with locally advanced stage III and stage IV cutaneous melanoma. The median overall survival was 17.8 months with the vaccine compared with 11.1 months with interleukin-2 alone (P=0.06) (15,16).
Gp100 was also tested in combination with ipilimumab, an antibody that blocks CTLA-4. The 3-year survival rate with ipilimumab alone was 25% and only 15% with the combination of gp100 and ipilimumab (16).
Vitespen is an autologous vaccine composed of tumor-derived heat shock proteins-peptide complexes. This vaccine was studied in a phase II clinical trial in stage IV melanoma patients. The overall survival in the vaccine treated patients was similar to that in patients treated with physician’s choice (17).
Ex vivo loading of dendritic cells with peptide has also been tested in a phase III clinical trial in metastatic melanoma. The vaccinated arm was compared with standard dacarbazine. No significant differences in the objective response were observed (18).
The lack of efficacy of these large clinical trials focused on traditional tumor-associated antigens has promoted the search for alternative strategies to elicit an immune response.
Personalized neoantigen-based vaccines
High mutation burden has shown a strong correlation with the clinical benefit in checkpoint inhibitor therapy (PD-L1/PD-1 and CTLA-4) (19-21) and several studies have associated the efficacy of cancer immunotherapy with the recognition of specific neoantigen by T cells in melanoma patients (22-24). Moreover, melanoma has the highest mutation load among the different cancers (25) which provides a large source of neoantigens that can be suitable for developing personalized vaccines.
Neoantigens are the result of tumor-specific DNA alterations that create new neoepitopes. These neoepitopes can be presented by antigen presenting cells to prime CD4+ and CD8+ lymphocytes. As compared with others tumor-associated antigens, which are non-mutated proteins, neoantigens are tumor-specific and have not been affected by central T cell tolerance (26).
Neoantigen-based vaccines need to be personalized because the set of cancer mutations in patients varies considerably. Briefly, neoantigen identification starts with exon sequencing from a tumor biopsy. When available, RNA data expressions are used to focus on the gene alterations that are expressed. Prediction algorithms allow the selection of those neoantigens presented in the class I or class II MHC complex. Finally, in vitro and in vivo approaches are used to validate the capacity of the neoantigens to stimulate either CD8+ or CD4+ T cell recognition thus allowing an anti-tumor response (Figure 1).
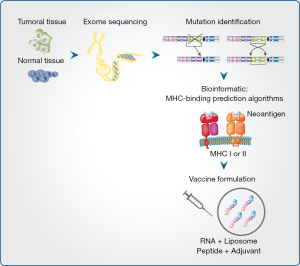
Currently, different strategies to express and delivery neoantigens are being evaluated in clinical trials (Table 1). Peptides containing neoantigens have been demonstrated to induce a strong immune response allowing control of tumor growth after systemic administration in mouse models (27). Currently, a clinical trial carried out by Neon Therapeutics in collaboration with Bristol-Myers Squibb is evaluating the efficacy of a personalized cancer vaccine (NEO-PV-01) in combination with an anti-CTLA4 in melanoma, lung, and bladder cancer patients (NCT02897765). Recently, the Dana-Farber Cancer Institute evaluated the efficacy of a personalized vaccine peptides co-administered with poly ICLC (NeoVax) (NCT01970358). The personalized vaccine that targets up 20 neoantigens was administrated in six melanoma patients after surgery to remove their tumors. Two years after treatment, four patients remain tumor-free. In the others two patients, the tumor reappeared. However, both patients reported a complete tumor regression after anti-PD1 treatment (28).

Full table
Neoantigens can also be provided ex vivo to antigen presenting cells. In 2015, a clinical trial using personalized tumor neoantigens was assessed in three melanoma patients (29). Autologous dendritic cells derived from patient monocytes were exposed to peptides containing neoantigens and then, neoantigen-loaded mature dendritic cells were injected back into the patient. T cell recognition and activation were observed. However, the clinical efficacy of the procedure has not yet been reported.
Systemic administration of naked RNA or a nanoparticle RNA vaccine expressing tumor neoantigens has shown promising efficacy in melanoma mice models (30,31). By modulating the net charge in the lipid carrier, nanoparticles could reach the antigen presenting cells in vivo and trigger IFNα release to promote a strong effector T-cell response and antitumor efficacy. Recently, a direct intranodal administration of the naked personalized RNA vaccine was evaluated in a phase 1 clinical trial (NCT02035956). A personalized RNA-based vaccine encodes up 10 neoantigens was administrated in 13 melanoma patients. Eight patients remain tumor-free one year after treatment. Five patients experienced tumor relapses during treatment. However, two patients reported tumor size reduction and one patient showed a complete response after anti-PD-1 administration (32).
Bioinformatic algorithms to predict the best neoantigens presented in MHC complex molecules and those which can be recognized by the TCR repertoire is a challenge. The so-called Tumor neoantigEn SeLection Alliance (TESLA) is a new approach focusing on neoantigens that include 30 of the world’s leading cancer neoantigen research groups from both academia and industry. TESLA mainly focuses on improving algorithms to analyze tumor DNA and RNA sequences in order to predict the best neoantigens that are going to be presented by APC in each patient.
Re-engineered viral vector or attenuated bioengineered Listeria monocytogenes bacteria have the potential for delivering multiple neoantigens and therefore, eliminate the need for MHC complex binding prediction algorithms. Currently, these strategies are being developed by two companies, and they are expected to enter the clinic setting in 2017.
Conclusions and future perspectives
Antitumor vaccines have been the focus of intense preclinical and clinical research and melanoma has been the focus of many of these efforts. Great advances have been made in delineating the basic principles for antitumor vaccine design. An impressive arsenal of vaccine vectors and adjuvants has been developed. However, clinical efficacy remains elusive. Personalized neoantigen vaccines hold great promise and several companies and academic centers are initiating clinical trials. On-going clinical trials will tell if neoantigens are the key for the clinical success of antitumor vaccines.
Acknowledgements
Funding: This work was supported by the grant PI13/00207 and PI16/00668 from Instituto de Salud Carlos III, financed by the FEDER program of the European Union, by a grant from the FAECC and by the EC’s H2020 PROCROP project, under grant agreement 635122. P Berraondo was supported by a Miguel Servet and Miguel Servet II (CPII15/00004) contract from Instituto de Salud Carlos III.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Galluzzi L, Buqué A, Kepp O, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017;17:97-111. [Crossref] [PubMed]
- Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013;13:525-41. [Crossref] [PubMed]
- Spitzer MH, Carmi Y, Reticker-Flynn NE, et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 2017;168:487-502.e15. [Crossref] [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [Crossref] [PubMed]
- Gonzalez-Aparicio M, Alzuguren P, Mauleon I, et al. Oxaliplatin in combination with liver-specific expression of interleukin 12 reduces the immunosuppressive microenvironment of tumours and eradicates metastatic colorectal cancer in mice. Gut 2011;60:341-9. [Crossref] [PubMed]
- Medina-Echeverz J, Fioravanti J, Zabala M, et al. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol 2011;186:807-15. [Crossref] [PubMed]
- Rodrigo-Garzón M, Berraondo P, Ochoa L, et al. Antitumoral efficacy of DNA nanoparticles in murine models of lung cancer and pulmonary metastasis. Cancer Gene Ther 2010;17:20-7. [Crossref] [PubMed]
- Alexandrescu DT, Ichim TE, Riordan NH, et al. Immunotherapy for melanoma: current status and perspectives. J Immunother 2010;33:570-90. [Crossref] [PubMed]
- Berraondo P, Nouzé C, Préville X, et al. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res 2007;67:8847-55. [Crossref] [PubMed]
- Corrales L, Glickman LH, McWhirter SM, et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep 2015;11:1018-30. [Crossref] [PubMed]
- Quetglas JI, Fioravanti J, Ardaiz N, et al. A Semliki forest virus vector engineered to express IFNα induces efficient elimination of established tumors. Gene Ther 2012;19:271-8. [Crossref] [PubMed]
- Vasquez M, Paredes-Cervantes V, Aranda F, et al. Antitumor effect of an adeno-associated virus expressing apolipoprotein A-1 fused to interferon alpha in an interferon alpha-resistant murine tumor model. Oncotarget 2017;8:5247-55. [PubMed]
- Mansilla C, Berraondo P, Durantez M, et al. Eradication of large tumors expressing human papillomavirus E7 protein by therapeutic vaccination with E7 fused to the extra domain a from fibronectin. Int J Cancer 2012;131:641-51. [Crossref] [PubMed]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004;10:909-15. [Crossref] [PubMed]
- Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011;364:2119-27. [Crossref] [PubMed]
- McDermott D, Haanen J, Chen TT, et al. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann Oncol 2013;24:2694-8. [Crossref] [PubMed]
- Testori A, Richards J, Whitman E, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol 2008;26:955-62. [Crossref] [PubMed]
- Schadendorf D, Ugurel S, Schuler-Thurner B, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol 2006;17:563-70. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015;350:207-11. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 2013;31:e439-42. [Crossref] [PubMed]
- Robbins PF, Lu YC, El-Gamil M, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 2013;19:747-52. [Crossref] [PubMed]
- Prickett TD, Crystal JS, Cohen CJ, et al. Durable Complete Response from Metastatic Melanoma after Transfer of Autologous T Cells Recognizing 10 Mutated Tumor Antigens. Cancer Immunol Res 2016;4:669-78. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [Crossref] [PubMed]
- Castle JC, Kreiter S, Diekmann J, et al. Exploiting the mutanome for tumor vaccination. Cancer Res 2012;72:1081-91. [Crossref] [PubMed]
- Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547:217-21. [Crossref] [PubMed]
- Carreno BM, Magrini V, Becker-Hapak M, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015;348:803-8. [Crossref] [PubMed]
- Kranz LM, Diken M, Haas H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016;534:396-401. [Crossref] [PubMed]
- Kreiter S, Vormehr M, van de Roemer N, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015;520:692-6. [Crossref] [PubMed]
- Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017;547:222-6. [Crossref] [PubMed]


