Surgical treatment of liver metastases in patients with neuroendocrine tumors
Introduction
Neuroendocrine tumors (NETs) are slow growing heterogeneous neoplasms, which are generally viewed with a favorable prognosis. This group of heterogeneous neoplasms defined as either non- functioning tumors often associated with liver metastases at the time of diagnosis, or functioning tumors that secrete peptide hormones. These hormones could cause characteristic patterns of symptoms, like flushing, diarrhea, and palpitation. NETs are comparatively uncommon, with an incidence range from 2.5 to 5.3 per 100,000 (1). Primarily, NETs arise from the gastro-entero-pancreatic neuroendocrine tract. Gastro-entero-pancreatic neuroendocrine tumors (GEP-NETs) can be either carcinoid tumors, which arise from the endocrine cells of the gastrointestinal tract, or pancreatic neuroendocrine tumors (2).
In 2010, World Health Organization (WHO) and Tumor Node Metastases (TNM) staging system of the European Neuroendocrine Tumor society (ENETS) and American Joint Committee on Cancer (AJCC) acknowledged a new classification system which identified three classes of tumors (G1, G2, G3) as defined by proliferative index (assessed by mitotic index and/or Ki 67). While the majority of NETs are recognized as well-differentiated tumors (G1, G2), neuroendocrine carcinomas belong to G3 category (3).
After the lymph nodes, the liver is the predominant site for NETs metastases. Synchronous liver metastases present in 75-80% of patients, which is a key adverse prognostic factor. When it is feasible, aggressive surgical management of both the primary tumor and the liver metastases improve overall survival rates extensively (4-6). Primary hepatic neuroendocrine tumors are extremely rare, and are diagnosed by exclusion of other primary tumors. As with metastases, the main treatment of primary hepatic NETs is surgical resection (7,8).
Currently, there are many therapeutic options for metastatic NETs. This includes surgery (e.g., open resection, laparoscopic resection, liver transplant), medical therapy (e.g., chemotherapy, biotherapy with somatostatin analogues and interferon, thermal ablative techniques (e.g., radiofrequency ablation (RFA), microwave ablation, cryotherapy) and embolization using transcatheter embolization, chemoembolization, or radioembolization.
RFA is a palliative option aiming at debulking and controlling hormonal symptoms. Accordingly, Laparoscopic RFA was suggested when other treatment modalities including chemotherapy, somatostatin analogues, chemoembolization, and resection failed. Akyildiz et al. reported one of the largest prospective experiences with radiofrequency ablation of neuroendocrine liver metastases. Akyildiz recommended selection criteria for this study include maximum tumor size of 10 cm, maximum number of tumors of fifteen, and less than 20% liver involvement. Symptom relief was achieved in 97% of all patients treated with laparoscopic RFA (9). Additionally, Berber et Siperstein demonstrated that there was no significant increase in the morbidity with repeat ablation cases. This supports the concept that laparoscopic RFA can be performed in a repeated fashion in the case of recurrence (10).
In addition to histopathological analysis and clinical assessment, biochemical profile plays a major role in the NETs diagnosis. Serum 5-hydroxyindoleactic acid (5-HIAA) a product of serotonin breakdown can be measured in a urine sample obtained over 24 hours, it is highly specific for NETs. However, 5-HIAA levels insensitivity necessitate measurement of other circulating peptide hormones such as Chromogranin A, Chromogranin A is 100% specific and highly sensitive marker for NETs (11-13). On the other hand, specific tumors are characterized with specific hormones such as pancreatic insulinoma, which is associated with elevated levels of Insulin and c-peptide; this is true as well in case of gastrinoma and Gastrin. Other markers are common to all NETs, such as synaptophysin, neuron-specific enolase (NSE) and calcitonin (4,14,15).
Imaging techniques have a significant role in the diagnosis and management of patients with liver metastases, this includes somatostatin receptor scintigraphy, CT scan and MR imaging. These techniques help in detecting the presence of liver metastases estimating the mass characteristics, distribution, and location to major vessels. Unfortunately, all of these techniques were unsuccessful to identify undersized liver metastases (i.e., tumors <0.5 cm in diameter).
This discussion focuses on surgical treatments of NETs liver metastases and examines all forms of surgical resection, as well as liver transplantation.
Open surgical resection
In general, NETs patients with liver metastasis present in one of two manners: (I) considerable liver disease with carcinoid syndrome in need of debulking or (II) limited disease potentially curable with aggressive curative resection. Numerous studies have confirmed complete hepatic resection for liver metastases has significantly improved long-term survival compared to other conservative treatments (5,6,16-18).
Aggressive surgical resection increases the 5-year survival of NETs with solitary liver metastasis to 100%. Where disseminated metastatic NETs suffer a 51% 5-year survival rate after surgical resection (19). Multiple factors including primary tumor site, histological grade and metastatic sites other than liver play a major role in the overall survival (20,21). The surgical approach used depends on the distribution of metastases. In unilobar metastasis, resection of the primary tumor and liver metastasis can be completed synchronously, while bilobar metastasis often requires incomplete left lobe resection and right portal vein ligation, followed by right lobe resection in a two-step approach (22).
Resection of the primary tumor and the adjacent mesenteric lymph nodes confer significant increases in the survival rate. The principle concern over aggressive surgery is patient safety, hepatectomy in conjunction with abdominal resections can be associated with significant morbidity and mortality especially synchronous pancreatectomy (23). Improved survival has been reported by Hill et al. when liver resection is coupled with pancreatic NETs resection. However this study has been criticized for significant selection bias (24).
Significant reductions in biomarkers are associated with symptoms relief and disease control. However, in case of multiple non-resectable hepatic metastases, no clinical trial advocates surgery over other modalities. Additionally, disease recurrence was reported after hepatic metastasis resection (25).
Operative management with SSAs is indicated to avoid intra and post-operative carcinoid crisis. This is especially critical in the case of metastatic functioning tumors often characterized by carcinoid syndrome (26).
Prophylactic cholecystectomy during abdominal exploration is indicated in NETs patients with liver metastases. This strategy is indicated to avoid gallstones associated with somatostatin long-term treatment, and prevent gallbladder necrosis due to hepatic artery embolization for liver metastases (27).
Minimally invasive laparoscopic surgery
Obesity and fatty liver are often considered major obstacles in metastatic hepatic NETs surgical resection. Metastatic lesions are frequently numerous and extremely vascular, more than 90% of liver Metastatic lesions are supplied by the hepatic artery, making resection more technically challenging. This might explain the trend toward less invasive approaches to metastatic liver NET tumors such as arterial embolization (28,29).
Laparoscopic liver resection has become the preferred surgical approach for many surgeons because of shorter operative time, decreased blood loss, and lesser blood transfusion, moreover, overall hospital stay was also drastically reduced, proving an earlier improvement and resumption of physiological functions (30-32). This is most notable in obese patients. Secondly, laparoscopic hepatectomy has been shown to be oncologically sound with equivalence to open surgery (32).
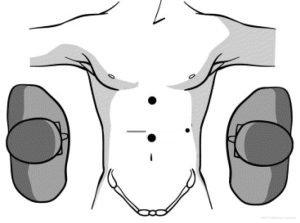
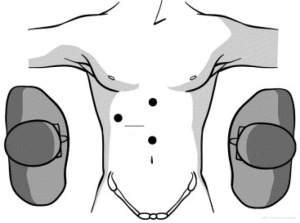
In laparoscopic liver resection the surgeons should maintain positions on opposite sides of the patient. In the case of left-sided tumors, the primary surgeon is on the patient’s right side. This position allows the primary surgeon to place his right hand under the left lateral segment, which allows inferior retraction of the liver for incision and division of the coronary attachments. The hand-assist device is inserted in the right midabdominal quadrant adjacent to the midline port (Figure 1). During right hepatic resections, hand-assist port placement is in a more superior and lateral position, and the surgeon is positioned on the patient’s left side and inserts his left hand (Figure 2). Selective vascular isolation is achieved through the stapler hepatectomy. During resection, the liver capsule is incised and the thickness of the parenchyma reduced with an ultrasonic dissector or tissuelink device (Figure 3). Once the initial 2 cm of the parenchyma is incised, the remaining resection is completed with an endovascular stapler. Homeostasis is subsequently achieved with an argon beam and completed with the use of biologic glues.
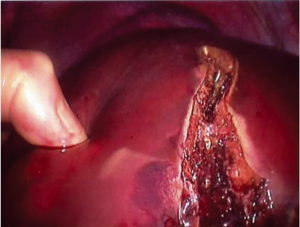
Recently, we have published our experience comparing laparoscopic to open liver resection for the management of NET liver metastases. Laparoscopic liver resection has a lower recurrence rate, 27% at mean interval of 14 months compared to 47% at a mean interval of 15.9 months in open resection. Laparoscopic resection has shorter operative time and hospital stays. The overall 3-year survival and 3-year disease free survival rate for the laparoscopic group 100% and 73.3%, respectively. Complications were lower in laparoscopic group compared to the open surgery group. In addition, laparoscopic surgery is considered safe and feasible in case of major, minor, atypical and even redo hepatectomy. However, metastases should not be more than four in number, and not require an extended hepatectomy to achieve negative margins (33).
Liver transplant
Liver transplantation should be considered another surgical option when both surgical and medical treatment fails to eradicate disease (34,35). Unfortunately, mortality after liver transplantations from recurrent liver disease remains a major concern. The five-year recurrence-free rates vary from 25-50% (36). Liver transplantation is a feasible option for young patients (<50 years old) with unresectable tumor, low ki-67 index and no extra-hepatic disease (15,37). Suspicious extra-hepatic lesions should be evaluated using exploratory laparoscopy or laparotomy prior to proceeding to liver transplant.
The primary tumor and lymph nodes must be resected before liver transplant. This will help to evade the high perioperative risks related to coupling of pancreatic or intestinal resections with the transplant operation (36,38). Furthermore, earlier primary tumor resection helps determining histopathological characteristics of the NET including Ki-67 index, and degree of differentiation, which are significant to patient selection for transplantation. Patients selection criteria includes patients with low grade tumor, G1, ten-percent or less Ki-67 index and 2 or less mitoses per high-power field (39).
Intractable carcinoid syndrome and hepatic failure Symptoms are all indications for liver transplantation. However, the selection criteria for transplantation should assure clinical improvement. Consequently, the improvement in patient’s quality of life must exceed the considerable risks of both the liver transplant and the immunosuppression (36,40,41).
Even though Chemotherapeutics, arterial embolization, and aggressive surgery for recurrent tumor may improve the survival rates (42), given the demand for donor organs and the need of fair selection criteria, liver transplantation is controversial (41).
Conclusions
There are a number of surgical options available for the treatment of NETs liver metastases. The choice of treatment depends on the symptoms, distribution of the metastases, and the histological features of the tumor. Nevertheless, there is no evidence-based data comparing surgery versus other liver-directed treatment options such as thermal ablation techniques, embolization, and somatostatin analogues in the management of patients with metastatic NETs. The future appears more encouraging with variable treatment options. Although aggressive surgical resection remains the gold standard for management, the laparoscopic option by experienced laparoscopic liver surgeons can be safe, feasible and provides earlier recovery and fewer complications. However, patients should be managed under the supervision of a multidisciplinary team to guarantee that all treatment options are explored both at diagnosis and follow-up.
Acknowledgements
Disclosure: (I) All financial and material support for this research and work was fully supported by Tulane University and Tulane University Hospital; (II) The authors have no financial interests in companies or other entities that have an interest in the information included in the contribution; (III) Additionally, there are no other conflicts of interest to report.
References
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72.
- Rossi RE, Massironi S, Spampatti MP, et al. Treatment of liver metastases in patients with digestive neuroendocrine tumors. J Gastrointest Surg 2012;16:1981-92.
- Rindi G, Arnold R, Bosman FT, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours of the Digestive System. France: International Agency for Research on cancer (IARC), Lyon 2010. IARS, 2010:S1-S14.
- Basuroy R, Srirajaskanthan R, Ramage JK. A multimodal approach to the management of neuroendocrine tumour liver metastases. Int J Hepatol 2012;2012:819193.
- Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190:432-45.
- Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg 2003;197:29-37.
- Huang YQ, Xu F, Yang JM, et al. Primary hepatic neuroendocrine carcinoma: clinical analysis of 11 cases. Hepatobiliary Pancreat Dis Int 2010;9:44-8.
- Yalav O, Ülkü A, Akçam TA, et al. Primary hepatic neuroendocrine tumor: Five cases with different preoperative diagnoses. Turk J Gastroenterol 2012;23:272-8.
- Akyildiz HY, Mitchell J, Milas M, et al. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up. Surgery 2010;148:1288-93; discussion 1293.
- Berber E, Siperstein AE. Perioperative outcome after laparoscopic radiofrequency ablation of liver tumors: an analysis of 521 cases. Surg Endosc 2007;21:613-8.
- O’Connor DT, Pandlan MR, Carlton E, et al. Rapid radioimmunoassay of circulating chromogranin A: in vitro stability, exploration of the neuroendocrine character of neoplasia, and assessment of the effects of organ failure. Clin Chem 1989;35:1631-7.
- O’Connor DT, Deftos LJ. Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med 1986;314:1145-51.
- Eriksson B, Arnberg H, Oberg K, et al. A polyclonal antiserum against chromogranin A and B--a new sensitive marker for neuroendocrine tumours. Acta Endocrinol (Copenh) 1990;122:145-55.
- Oberg K, Stridsberg M. Chromogranins as diagnostic and prognostic markers in neuroendocrine tumours. Adv Exp Med Biol 2000;482:329-37.
- Steinmüller T, Kianmanesh R, Falconi M, et al. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2008;87:47-62.
- Frilling A, Sotiropoulos GC, Li J, et al. Multimodal management of neuroendocrine liver metastases. HPB (Oxford) 2010;12:361-79.
- Mayo SC, de Jong MC, Bloomston M, et al. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol 2011;18:3657-65.
- Chen H, Hardacre JM, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg 1998;187:88-92; discussion 92-3.
- Frilling A, Li J, Malamutmann E, et al. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br J Surg 2009;96:175-84.
- Saxena A, Chua TC, Sarkar A, et al. Progression and survival results after radical hepatic metastasectomy of indolent advanced neuroendocrine neoplasms (NENs) supports an aggressive surgical approach. Surgery 2011;149:209-20.
- Cho CS, Labow DM, Tang L, et al. Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer 2008;113:126-34.
- Kianmanesh R, Sauvanet A, Hentic O, et al. Two-step surgery for synchronous bilobar liver metastases from digestive endocrine tumors: a safe approach for radical resection. Ann Surg 2008;247:659-65.
- D’Angelica M, Martin RC 2nd, Jarnagin WR, et al. Major hepatectomy with simultaneous pancreatectomy for advanced hepatobiliary cancer. J Am Coll Surg 2004;198:570-6.
- Hill JS, McPhee JT, McDade TP, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer 2009;115:741-51.
- Dousset B, Saint-Marc O, Pitre J, et al. Metastatic endocrine tumors: medical treatment, surgical resection, or liver transplantation. World J Surg 1996;20:908-14; discussion 914-5.
- Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 2004;15:966-73.
- Benkel M, Brasch F, Neumann JD, et al. Poorly differentiated neuroendocrine small-cell carcinoma of the gallbladder. Zentralbl Chir 2012;137:71-2.
- Harring TR, Nguyen NT, Goss JA, et al. Treatment of liver metastases in patients with neuroendocrine tumors: a comprehensive review. Int J Hepatol 2011;2011:154541.
- Atwell TD, Charboneau JW, Que FG, et al. Treatment of neuroendocrine cancer metastatic to the liver: the role of ablative techniques. Cardiovasc Intervent Radiol 2005;28:409-21.
- Morino M, Morra I, Rosso E, et al. Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc 2003;17:1914-8.
- Polignano FM, Quyn AJ, de Figueiredo RS, et al. Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc 2008;22:2564-70.
- Buell JF, Thomas MT, Rudich S, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg 2008;248:475-86.
- Kandil E, Noureldine SI, Koffron A, et al. Outcomes of laparoscopic and open resection for neuroendocrine liver metastases. Surgery 2012;152:1225-31.
- Ramage JK, Ahmed A, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 2012;61:6-32.
- Grossman EJ, Millis JM. Liver transplantation for non-hepatocellular carcinoma malignancy: Indications, limitations, and analysis of the current literature. Liver Transpl 2010;16:930-42.
- Le Treut YP, Grégoire E, Belghiti J, et al. Predictors of long-term survival after liver transplantation for metastatic endocrine tumors: an 85-case French multicentric report. Am J Transplant 2008;8:1205-13.
- Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157-76.
- Máthé Z, Tagkalos E, Paul A, et al. Liver transplantation for hepatic metastases of neuroendocrine pancreatic tumors: a survival-based analysis. Transplantation 2011;91:575-82.
- Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707-12.
- Lang H, Oldhafer KJ, Weimann A, et al. Liver transplantation for metastatic neuroendocrine tumors. Ann Surg 1997;225:347-54.
- Frilling A, Malago M, Weber F, et al. Liver transplantation for patients with metastatic endocrine tumors: single-center experience with 15 patients. Liver Transpl 2006;12:1089-96.
- Gregoire E, Le Treut YP. Liver transplantation for primary or secondary endocrine tumors. Transpl Int 2010;23:704-11.


