Inhalation therapies in acute respiratory distress syndrome
Introduction
In acute respiratory distress syndrome (ARDS), overproduction of inflammatory factors in lung tissue is followed by pulmonary edema and severe hypoxemia and an increase in pulmonary dead space. Despite significant advances in our understanding and management of patients with ARDS, the morbidity and mortality associated with ARDS remains high. Few measures have been proven to improve outcomes in ARDS. Treatment consists mainly of measures to avoid worsening lung injury, such as lung-protective mechanical ventilation (1), prone positioning (2), and neuromuscular blockers (3); these are the only strategies that proved effective in reducing mortality. However, these approaches are unable to reverse the pathophysiological processes that underlie ARDS.
ARDS can result from direct or indirect insult, such as infection or trauma. Specific hallmarks of the disease include dysregulation of inflammation, accumulation of leukocytes and platelets with the activation of coagulation pathways, and disruption of the endothelial and epithelial barriers in the alveoli increasing their permeability to proteins (4). Decades of research have failed to find effective therapies that reduce mortality in established ARDS, and preclinical studies suggest that therapies that prevent lung injury when employed before the injury have lesser or no beneficial effects after lung injury develops (5). In recent years, new pharmacological agents designed to decrease the release of pro-inflammatory cytokines have shown promising results in preclinical studies; moreover, these studies have furthered our understanding of the mechanisms involved in the pathogenesis and resolution of lung injury (6). However, clinical studies have failed to extend these results to humans and no pharmacological treatment for ARDS has been successful in a controlled trial (7).
Given that relatively few therapeutic measures have proven effective in ARDS, intravenous treatments can result in systemic side effects, and early treatment is crucial in critically ill patients, the focus has started to shift toward seeking strategies to prevent ARDS. One such strategy aims to develop local pulmonary treatments that deliver medications directly to the lungs by nebulization, with the aim of increasing local efficacy and minimizing systemic adverse effects.
This review discusses inhalational therapies (aerosols or gases) in the prevention and treatment of ARDS.
Aerosol delivery in mechanical ventilated patients
The medical use of aerosols dates back to ancient times (8). Two factors make therapeutic aerosols inherently attractive. Drugs can be delivered directly to the respiratory tree, to the alveolar epithelium, or both; moreover, the lungs’ huge capacity for absorption and diffusion ensures that inhaled drugs reach the systemic circulation quickly.
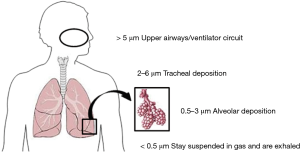
Aerosols are colloidal suspensions of particles in gas. The size, shape, and density of particles together with the density and viscosity of the gas determine the extent to which particles can remain suspended. Two variables are often used to characterize aerosols. The first, the mass median aerodynamic diameter reflects the size of the particles; whereas the second, the geometric standard deviation, reflects the degree of variation in the size of the particles. These properties make it possible to predict where the particles will be deposited within the tracheobronchial tree/ventilator circuit (Figure 1). The efficiency of an aerosol generator can be determined if the rate of particle production and the geometric standard deviation are known. The ventilator circuit and settings have a bigger impact than the aerosol generator on time required for drugs to reach their targets and on the proportion of drugs that reach these targets.
In patients receiving mechanical ventilation, it can be a challenge to ensure that a generator delivers aerosolized particles to distal airways to reach the alveoli, and the proportion of the drug that reaches the target site depends on various factors (Table 1). Delivery depends on the ventilator settings and the physiological/pathophysiological factors of the patient’s airflow. To ensure peripheral drug deposition, the ventilator should be set for: (I) low bias flow; (II) higher tidal volume and/or recruitment maneuver to distribute the drug more extensively; (III) a long, slow, continuous inspiratory profile; (IV) a long pause at end-inspiration to maximize the impaction/dropout of particles in peripheral regions; and (V) positive end-expiratory pressure (PEEP) to prevent alveolar collapse during expiration. Modern ventilators all synchronize aerosol production with inspiration to improve drug delivery (9).

Full table
Aerosolized drugs
Bronchodilators
In patients undergoing mechanical ventilation, the drug most often prescribed for nebulization is albuterol (racemic salbutamol). This β2-agonist is not only a bronchodilator; it also seems to improve fluid clearance (10,11) and favor mucociliary clearance (12).
Most patients with ARDS have impaired fluid clearance from the alveoli, and increased impairment is associated with increased mortality (13). Alveolar fluid clearance can be improved by stimulating pulmonary β2-adrenergic receptors, which upregulates and activates sodium and chloride transport proteins through cyclic adenosine monophosphate (14). One study in mice with lung injury due to the aspiration of acid found that clinically relevant concentrations of β2-agonists resulted in clearance of fluid from alveoli dependent on cyclic adenosine monophosphate and decreased pulmonary edema (15). Albuterol levels reached therapeutic levels in the pulmonary edema fluid of mechanically ventilated ARDS patients (16). More importantly, inhaling salmeterol prophylaxis prevents high-altitude pulmonary edema (17).
However, two randomized multicenter trials testing selective β2-agonists for established ARDS found that these drugs not only failed to provide clinical benefits but actually seemed to worsen outcomes when given intravenously (18,19). Thus, it may be that β2-agonists increase fluid clearance only in patients without damage to the alveolar epithelium before the onset of ARDS. Perkins et al. (20) studied 362 patients undergoing esophagectomy in 12 centers in the United Kingdom to determine whether inhaling salmeterol could prevent ARDS. They found no difference in the incidence of ARDS between salmeterol and placebo (OR, 1.25; 95% CI, 0.71–2.22), but postoperative adverse events (mainly pneumonia) were less frequent in patients receiving salmeterol. A sub-study of 53 patients found that several biomarkers of alveolar inflammation and epithelial injury were lower in patients receiving salmeterol.
Despite extensive, supportive preclinical data and sound physiologic rationale, clinical trials have failed to improve outcome in established ARDS, but why? There are several plausible explanations, including lack of fidelity in preclinical models, substantial heterogeneity of ARDS in human trials, and changes in critical care practices (conservative fluid management and lower tidal volumes). However, timing of intervention and suboptimal dosing may be most critical, as data from preclinical and observational studies suggest.
Recently, Festic et al. (21) took an important first step toward answering this question with the “Lung Injury Prevention Study with Budesonide and Beta agonist (LIPS-B)” phase II randomized clinical trial comparing blinded treatment with aerosolized corticosteroids (budesonide 0.5 mg/2 mL) in combination with a long-acting beta agonist (formoterol 20 mcg/2 mL) to placebo administered twice daily during 5 days for ARDS prevention among at-risk patients. Patients were approached for consent in the emergency department and the treatment was administered within 9 hours, demonstrating that the early intervention for ARDS prevention is feasible. Standardization of ICU best practices was recommended and the most common reasons for exclusion were the use of steroids or β-agonists prior to enrollment and inability to obtain consent within 12 hours. The high rate of exclusion, including smokers with chronic obstructive pulmonary disease who often use inhaled steroids and β-agonists, could limit the generalizability of the study; this is particularly important given that smokers have a higher risk of developing ARDS. The investigators found a significant improvement in oxygenation as measure by the oxygen saturation divided by inspired oxygen fraction (S/F ratio) in the group treated with inhaled budesonide/formoterol that became evident on days 2 and 4 of the protocol, but did not persist to day 5. Secondary outcomes included the development of ARDS, hospital and ICU length of stay, and the need for mechanical ventilation, which were not significantly different after adjusting for baseline differences in the rate of shock. Subjects in the treatment group appeared to be less acutely ill with lower LIPS and had less shock compared to subjects in the placebo group (13% vs. 47%). However, the improvement in oxygenation as measured by the S/F ratio remained statistically different between groups after logistic regression adjusting for LIPS or the presence of shock. In addition to the longitudinal improvement in oxygenation, the study demonstrated that it is feasible to rapidly enroll and safety treat these patients in the emergency department. These results support further studies to test the efficacy of inhaled corticosteroids and beta agonists for ARDS prevention. A phase 2 trial could address some of the limitations of the present study, such as unbalanced ARDS risk in the treatment arm, and could determine if more sustained improvements in gas exchange, survival, and ventilator-free days are achievable with the use of inhaled steroids and long-acting β-agonists in patients at risk of ARDS.
After salbutamol, ipratropium bromide, an anticholinergic bronchodilator, is the drug most commonly administered in mechanically ventilated patients by nebulization, even though, like salbutamol, its effectiveness has yet to be demonstrated in clinical trials.
ARDS survivors frequently present a decrease in expiration flow rate with airway hyperreactivity and air trapping due to small airways disease, indicating a need to maintain bronchodilator treatment for 6 months after hospital discharge (22).
Corticosteroids
Given the role of unregulated inflammation in ARDS, there has been interest in inhaled corticosteroids for treatment and prevention of ARDS in patients in whom systemic steroid administration may not be desirable (23-26). Corticosteroids have proven beneficial in many lung injuries of different origins, such as diffuse alveolar hemorrhage, that progress to ARDS. Animal models of lung injury have consistently shown amelioration of histologic injury, and improvement in oxygenation and respiratory mechanics in animals treated with inhaled corticosteroids even with heterogeneity in timing treatment and mechanisms of inciting injury.
Although studies in patients with severe influenza pneumonia showed no benefit for corticosteroids, other studies in patients hospitalized with pneumonia found that systemic steroids reduced treatment failure, including in patients with ARDS (27-29). Although limited clinical data exist, a secondary analysis of the LIPS cohort found that the use of inhaled corticosteroids prior to hospitalization was associated with a decreased risk of developing ARDS (OR, 0.39; 95% CI, 0.14–0.93) (30). A recent trial demonstrated that nebulized budesonide (1 mg/2 mL) improved oxygenation and peak and plateau airway pressures, and significantly reduced inflammatory markers (TNFα, IL-1β, and IL6) without affecting hemodynamics (31).
However, point-of-care measurements will be needed to integrate with clinical criteria to identify patients who might benefit based on a more pro-inflammatory phenotype (32).
Pulmonary vasodilators
Inhaled nitric oxide (iNO) decreases pulmonary artery pressure and improves oxygenation significantly, but fails to reduce mortality in patients with ARDS no matter how severe their hypoxemia is; moreover, iNO might increase the risk of renal impairment (33). A recent meta-analysis of 1,142 patients in nine homogeneous randomized trials found no reduction in mortality in patients with severe ARDS who received iNO (RR, 1.01; 95% CI, 0.78–1.32) (34). Furthermore, analyzing subgroups of patients with PaO2/FIO2 ratios (70–200 mmHg) failed to find a cutoff for which iNO reduces mortality. A retrospective study carried out in a single center that examined the effectiveness, safety, and cost of inhaled epoprostenol (iEPO) versus iNO in 105 patients found no differences between the two treatments in several clinical and outcome measures (35). The greatest benefits for iEPO have been reported in ARDS patients with right ventricular heart failure at the outset. A potential effect of inhaled prostaglandins could be derived from their antiplatelet and anti-inflammatory properties, but further studies are needed to analyze their impact on outcome (36).
In surveys, 29% to 44% of intensivists from UK and Germany report administering iNO in ARDS (37,38). However, the LUNG SAFE study found that physicians prescribed inhaled vasodilators much less often: only 7.7% of all patients with ARDS and only 13.0% of those with severe ARDS received these drugs (39). Although inhaled vasodilators improve hemodynamic parameters and oxygenation in ARDS patients, they should be used mainly as a rescue therapy only after traditional treatments fail, rather than as standard care in ARDS.
Anticoagulants
Dysregulated coagulation, mediated by the tissue factor (TF) pathway, is another pathophysiological hallmark of ARDS, and agents targeting the coagulation cascade are putative candidates for ARDS treatment and prevention. Pulmonary coagulation is evident in increased markers of thrombin generation, soluble TF, and factor VIIa activity found in bronchoalveolar lavage fluid from ARDS patients, together with an increased release of plasminogen activator inhibitor-1 (PAI-1) decreasing fibrinolytic activity (40). The deposition of intravascular and extravascular fibrin as a result of activated coagulation and impaired fibrinolysis may contribute to lung inflammation, endothelial cell activation, and disruption of the alveolar capillary membrane barrier. Local administration of nebulized anticoagulants to the lungs allows higher dosages and increases local efficacy, reduces the risk of systemic bleeding, and is more effective than intravenous administration (41). In addition to its anticoagulant activity, intravenously administered heparin had anti-inflammatory effects, ameliorating the injury induced by lipopolysaccharide in a model of acute lung injury (ALI) (42). Heparin reduces the expression of proinflammatory mediators in human alveolar macrophages injured by lipopolysaccharide and decreases the NF-kβ pathway in alveolar cells (43). Furthermore, nebulized heparin decreases pro-inflammatory cytokines in lung tissue and the expression of NF-kB and TGF-β effectors in alveolar macrophages (44).
In smoke inhalation-related lung injury, preclinical and clinical studies have suggested that administration of inhaled anticoagulants improves oxygenation, reduces lung injury severity, and improves survival without altering systemic markers of clotting and anticoagulation (45). In a clinical trial in 50 patients requiring extended mechanical ventilation for any reason, the group randomized to inhaled heparin required fewer days of mechanical ventilation compared to the placebo group, although no improvements in oxygenation or outcomes were observed (46). Hofstra et al. (47) showed that local treatment with recombinant human activated protein C (APC), plasma-derived antithrombin, heparin, and danaparoid attenuated pulmonary coagulopathy, but not inflammation, in rats with endotoxemia-induced lung injury. A recent multicenter trial to investigate the efficacy and safety of nebulized heparin (HEPBURN) in burn patients with inhalation trauma was stopped earlier due to adverse events and futility (48).
The protein C anticoagulant pathway limits the activation of homeostasis and has anti-inflammatory effects (49,50). Thus, the activation of this pathway in the alveolar epithelium could modulate the deposition of fibrin in the alveoli. Inhaled protein C also decreases the recruitment of neutrophils into airspaces, resulting in an antiapoptotic effect; this, together with its anticoagulant, profibrinolytic, and anti-inflammatory effects, make inhaled protein C ideal to counteract the pathophysiological changes seen in ARDS. Another advantage is that it appears that inhaled APC does not interfere with pulmonary host defense. Aerosolized APC might be optimal for treating ARDS because in this inflammatory lung condition the normal conversion of protein C into APC in the lungs is disrupted and targets in the distal airspaces mediate inhaled APC’s effects. Preclinical and limited clinical experience support this possibility (49,51,52). Unfortunately, this hypothesis cannot be tested in larger series of ARDS patients due to the negative PROWESS-Shock trial and the removal of APC from the market (53).
Mucolytic agents
Normal respiratory tract secretions are mainly made up of gel-forming mucin glycoproteins that form large oligomeric structures. Sputum or pathological respiratory mucus also contain other elements and have a much higher viscosity, which favors clearance through coughing but makes mucociliary clearance less efficient (54). In mechanically ventilated patients, both cough and mucociliary clearance are reduced, so treatments targeting sputum viscosity might be useful. N-acetylcysteine is the most widely recommended mucolytic, but its efficacy has yet to be confirmed for pathological condition (54,55). By contrast, strong evidence for the efficacy of nebulized hypertonic (3–14%) saline has been presented (54,56). Animal studies of nebulized hypertonic saline suggest that the preadministration of hypertonic saline attenuates the severity of lung injury, reduces matrix metalloproteinase activity, and decreases cytokine production from macrophages and epithelial cells (57,58). A phase I clinical trial is currently recruiting patients with post-traumatic ARDS to investigate nebulized hypertonic saline [registered with www.clinical.trials.gov (NTCO1667666)].
Surfactant
Pulmonary surfactant is produced by type II alveolar epithelial cells; it is composed of phospholipids, proteins, and neutral lipids. Surfactant plays important roles in maintaining alveolar surface tension and in the host immune response (59). Surfactants in ARDS patients’ bronchoalveolar lavage fluid have alterations in phospholipid composition and lower concentrations of surfactant proteins (60-62). Although exogenous surfactant confers clinical benefits in pediatric patients, several phase III clinical trials have failed to find beneficial effects in adult ARDS patients (63). However, these studies had various shortcomings, such as the failure to deliver enough surfactant or to incorporate hydrophilic surfactant proteins; moreover, these studies might not have target the groups of patients with the greatest probability of deriving benefits from this treatment. Newer approaches that assess surfactant synthesis and metabolism by stable isotope labeling of surfactant precursors (64) might make it possible to target patients who synthesize insufficient amounts of surfactant and are therefore likely benefit from treatment with exogenous surfactant. For the time being, however, no added value can be attributed to the use of exogenous surfactant in adult ARDS patients, although improved delivery methods may make it possible to retest surfactant in selected patients with ARDS such as aspiration and pneumonia.
Inhaled carbon monoxide (CO)
CO produced by inducible enzyme heme oxygenase-1 (HO-1) during heme catabolism functions as a signaling molecule. In response to cellular stress, the HO-1/CO system activates anti-inflammatory, antioxidant, and anti-apoptotic defensive mechanisms while stimulating mitochondrial quality control programs and biogenesis. Pharmacological activation of the HO-1/CO system by inhaling low doses of CO results in protective effects against inflammation, oxidative stress, ischemia/reperfusion injury, sepsis, lung inflammation, ALI, and other pathological conditions. Thus, low-dose inhaled CO might be useful in critically ill patients, especially in those with sepsis and pneumonia-induced ARDS. However, some hurdles must be overcome before low-dose inhaled CO can be routinely used in patients; for instance, a ventilator-compatible system to deliver the gas and a safe, evidence-based dosing strategy must be devised. Fredenburgh et al. (65) proved that inhaled CO gas can be administered safely during mechanical ventilation; their clinically relevant nonhuman primate pneumonia model also provides preliminary evidence that this treatment might lead to earlier resolution of ALI by reducing extravascular fluid in the lungs. They found that inhaling CO at a concentration of 200 ppm for 60 minutes was able to achieve 6–8% COHb levels with ambient CO levels ≤1 ppm and was well tolerated. A phase II clinical trial is underway to test inhaled CO gas in critically ill patients with ARDS.
Novel peptide activating pulmonary edema clearance
Fluid homeostasis in the lung depends on Na+ ions being absorbed through apical epithelial sodium channels (ENaC). ENaC initiates transepithelial transport of Na+ ions on the surface and Na+/K+-ATPase drives excess fluid from the alveoli (14). Thus, activating ENaC is a useful approach to restore lung fluid homeostasis. Ware and Matthay (13) found that most ARDS patients have impaired alveolar fluid clearance and that maximal alveolar fluid clearance is associated with better clinical outcomes.
Recently, AP301, a synthetic peptide, has been reported to activate ENaC, promoting lung alveolar fluid clearance through a novel mechanism of ENaC activation. This peptide directly binds to intracellular carboxy-terminal of the α-subunit of ENaC, which increases the likelihood of the channel being open and thus enhances Na+ absorption (66,67). In a phase IIa randomized trial in 40 mechanically ventilated patients with pulmonary permeability edema, inhaled AP301 resulted in earlier and more pronounced reduction in extravascular lung water compared to placebo, indicating that the peptide activated alveolar clearance (68). A phase IIB/III trial will soon assess the safety and determine doses and efficacy for future phase III trials.
Future directions
Since the 1980s, several pharmacologic agents have failed in clinical trials for ARDS. Why have so many rationally chosen drugs proved ineffective? Reasons include the heterogeneity of underlying pathology, the heterogeneous patient population, and the lack of an ideal animal model and specific biomarkers for early diagnosis. ARDS faces three relatively unique pharmacological challenges: (I) ARDS patients are vulnerable due to concomitant multiple organ dysfunction, so they may not tolerate off-target effects of drugs; (II) inhaled drug delivery is impeded by the proteinaceous fluid in the injured alveoli and the inhomogeneous ventilation distribution where the damaged lung area is not ventilated; (III) ARDS is heterogeneous in its underlying pathophysiology, so targeting one pathway is unlikely to improve most patients’ outcomes. To find solutions for these three unique pharmacological challenges for the diverse ARDS population, a drug should concentrate in alveoli and target multiple alveolar cell types and cell processes. Nanomedicine primarily involves the use of nano-scale (usually 100 nm) drug carriers to improve the localization, kinetics, and sometimes pharmacodynamics of drugs. Pulmonary nanomedicine has the long-term potential to benefit nearly all lung diseases by increasing local concentrations of drugs in the lung and expanding the repertoire of drug formulations that can be used with attractive efficacy and safety profiles. Most nanomedicine-enhanced applications are likely to employ delivery by inhalation (69,70).
Acknowledgements
Funding: This work was supported by CIBERES and Fundació Parc Taulí, and partially by the research grant Instituto Carlos III PI12/02548. Dr. Matthay was supported by NHLBI HL51856.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [Crossref] [PubMed]
- Yadav H, Thompson BT, Gajic O. Fifty Years of Research in ARDS. Is Acute Respiratory Distress Syndrome a Preventable Disease? Am J Respir Crit Care Med 2017;195:725-36. [Crossref] [PubMed]
- Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 2006;27:337-49. [Crossref] [PubMed]
- Standiford TJ, Ward PA. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res 2016;167:183-91. [Crossref] [PubMed]
- Rubin BK. Air and soul: the science and application of aerosol therapy. Respir Care 2010;55:911-21. [PubMed]
- Ari A, Atalay OT, Harwood R, et al. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care 2010;55:845-51. [PubMed]
- Mutlu GM, Factor P. Alveolar epithelial beta2-adrenergic receptors. Am J Respir Cell Mol Biol 2008;38:127-34. [Crossref] [PubMed]
- Perkins GD, McAuley DF, Thickett DR, et al. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 2006;173:281-7. [Crossref] [PubMed]
- Restrepo RD. Inhaled adrenergics and anticholinergics in obstructive lung disease: do they enhance mucociliary clearance? Respir Care 2007;52:1159-73; discussion 1173-5. [PubMed]
- Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376-83. [Crossref] [PubMed]
- Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol 2006;35:10-9. [Crossref] [PubMed]
- McAuley DF, Frank JA, Fang X, et al. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med 2004;32:1470-6. [Crossref] [PubMed]
- Atabai K, Ware LB, Snider ME, et al. Aerosolized beta(2)-adrenergic agonists achieve therapeutic levels in the pulmonary edema fluid of ventilated patients with acute respiratory failure. Intensive Care Med 2002;28:705-11. [Crossref] [PubMed]
- Sartori C, Allemann Y, Duplain H, et al. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med 2002;346:1631-6. [Crossref] [PubMed]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Matthay MA, Brower RG, et al. Randomized, placebo-controlled clinical trial of an aerosolized β-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011;184:561-8.
- Gao Smith F, Perkins GD, Gates S, et al. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012;379:229-35. [Crossref] [PubMed]
- Perkins GD, Gates S, Park D, et al. The beta agonist lung injury trial prevention. A randomized controlled trial. Am J Respir Crit Care Med 2014;189:674-83. [Crossref] [PubMed]
- Festic E, Carr GE, Cartin-Ceba R, et al. Randomized Clinical Trial of a Combination of an Inhaled Corticosteroid and Beta Agonist in Patients at Risk of Developing the Acute Respiratory Distress Syndrome. Crit Care Med 2017;45:798-805. [Crossref] [PubMed]
- Evans TW, Griffiths MJ, Keogh BF. editors. ARDS. Sheffield: European Respiratory Society, 2002.
- Forsgren PE, Modig JA, Dahlbäck CM, et al. Prophylactic treatment with an aerosolized corticosteroid liposome in a porcine model of early ARDS induced by endotoxaemia. Acta Chir Scand 1990;156:423-31. [PubMed]
- Jansson AH, Eriksson C, Wang X. Effects of budesonide and N-acetylcysteine on acute lung hyperinflation, inflammation and injury in rats. Vascul Pharmacol 2005;43:101-11. [Crossref] [PubMed]
- Wang J, Zhang L, Walther SM. Administration of aerosolized terbutaline and budesonide reduces chlorine gas-induced acute lung injury. J Trauma 2004;56:850-862. [Crossref] [PubMed]
- Walther S, Jansson I, Berg S, et al. Pulmonary granulocyte accumulation is reduced by nebulized corticosteroid in septic pigs. Acta Anaesthesiol Scand 1992;36:651-5. [Crossref] [PubMed]
- Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677-86. [Crossref] [PubMed]
- Blum CA, Nigro N, Winzeler B, et al. Corticosteroid treatment for community-acquired pneumonia--the STEP trial: study protocol for a randomized controlled trial. Trials 2014;15:257. [Crossref] [PubMed]
- Siemieniuk RAC, Meade MO, Alonso-Coello P, et al. Corticosteroid Therapy for Patients Hospitalized With Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:519-28. [Crossref] [PubMed]
- Ortiz-Diaz E, Li G, Kor D, et al. Preadmission Use of Inhaled Corticosteroids Is Associated With a Reduced Risk of Direct Acute Lung Injury/Acute Respiratory Distress Syndrome. Chest 2011;140:912A. [Crossref]
- Mohamed HS, Meguid MM. Effect of nebulized budesonide on respiratory mechanics and oxygenation in acute lung injury/acute respiratory distress syndrome: Randomized controlled study. Saudi J Anaesth 2017;11:9-14. [Crossref] [PubMed]
- Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2:611-20. [Crossref] [PubMed]
- Afshari A, Brok J, Møller AM, et al. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Syst Rev 2010.CD002787. [PubMed]
- Adhikari NK, Dellinger RP, Lundin S, et al. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med 2014;42:404-12. [Crossref] [PubMed]
- Torbic H, Szumita PM, Anger KE, et al. Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. J Crit Care 2013;28:844-8. [Crossref] [PubMed]
- Fuller BM, Mohr NM, Skrupky L, et al. The use of inhaled prostaglandins in patients with ARDS: a systematic review and meta-analysis. Chest 2015;147:1510-22. [Crossref] [PubMed]
- Kredel M, Bierbaum D, Lotz C, et al. Therapy of acute respiratory distress syndrome : Survey of German ARDS centers and scientific evidence. Anaesthesist 2015;64:277-85. [Crossref] [PubMed]
- Dushianthan A, Cusack R, Chee N, et al. Perceptions of diagnosis and management of patients with acute respiratory distress syndrome: a survey of United Kingdom intensive care physicians. BMC Anesthesiol 2014;14:87. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Günther A, Mosavi P, Heinemann S, et al. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med 2000;161:454-62. [Crossref] [PubMed]
- Tuinman PR, Dixon B, Levi M, et al. Nebulized anticoagulants for acute lung injury - a systematic review of preclinical and clinical investigations. Crit Care 2012;16:R70. [Crossref] [PubMed]
- Li Y, Sun JF, Cui X, et al. The effect of heparin administration in animal models of sepsis: a prospective study in Escherichia coli-challenged mice and a systematic review and metaregression analysis of published studies. Crit Care Med 2011;39:1104-12. [Crossref] [PubMed]
- Camprubí-Rimblas M, Guillamat-Prats R, Lebouvier T, et al. Role of heparin in pulmonary cell populations in an in-vitro model of acute lung injury. Respir Res 2017;18:89. [Crossref] [PubMed]
- Camprubí-Rimblas M, Chimenti L, Guillamat-Prats R, et al. Heparin effect in alveolar macrophages in acute lung injury model. C105 respiratory failure: mechanistic insights from lung injury models. Am J Respir Crit Care Med 2016;193:A6295.
- Miller AC, Elamin EM, Suffredini AF. Inhaled anticoagulation regimens for the treatment of smoke inhalation-associated acute lung injury: a systematic review. Crit Care Med 2014;42:413-9. [Crossref] [PubMed]
- Dixon B, Schultz MJ, Smith R, et al. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: a randomized controlled trial. Crit Care 2010;14:R180. [Crossref] [PubMed]
- Hofstra JJ, Vlaar AP, Cornet AD, et al. Nebulized anticoagulants limit pulmonary coagulopathy, but not inflammation, in a model of experimental lung injury. J Aerosol Med Pulm Drug Deliv 2010;23:105-11. [Crossref] [PubMed]
- Glas GJ, Muller J, Binnekade JM, et al. HEPBURN - investigating the efficacy and safety of nebulized heparin versus placebo in burn patients with inhalation trauma: study protocol for a multi-center randomized controlled trial. Trials 2014;15:91. [Crossref] [PubMed]
- Heslet L, Andersen JS, Sengeløv H, et al. Inhalation of activated protein C: A possible new adjunctive intervention in acute respiratory distress syndrome. Biologics 2007;1:465-72. [PubMed]
- Puig F, Fuster G, Adda M, et al. Barrier-protective effects of activated protein C in human alveolar epithelial cells. PloS One 2013;8:e56965. [Crossref] [PubMed]
- Choi G, Hofstra JJ, Roelofs JJ, et al. Recombinant human activated protein C inhibits local and systemic activation of coagulation without influencing inflammation during Pseudomonas aeruginosa pneumonia in rats. Crit Care Med 2007;35:1362-8. [Crossref] [PubMed]
- van der Poll T, Levi M, Nick JA, et al. Activated protein C inhibits local coagulation after intrapulmonary delivery of endotoxin in humans. Am J Respir Crit Care Med 2005;171:1125-8. [Crossref] [PubMed]
- Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 2012;366:2055-64. [Crossref] [PubMed]
- Rogers DF. Mucoactive agents for airway mucus hypersecretory diseases. Respir Care 2007;52:1176-93; discussion 1193-7. [PubMed]
- Nash EF, Stephenson A, Ratjen F, et al. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev 2009.CD007168. [PubMed]
- Wark P, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev 2009.CD001506. [PubMed]
- Cuschieri J, Gourlay D, Garcia I, et al. Hypertonic preconditioning inhibits macrophage responsiveness to endotoxin. J Immunol 2002;168:1389-96. [Crossref] [PubMed]
- Wohlauer M, Moore EE, Silliman CC, et al. Nebulized hypertonic saline attenuates acute lung injury following trauma and hemorrhagic shock via inhibition of matrix metalloproteinase-13. Crit Care Med 2012;40:2647-53. [Crossref] [PubMed]
- Hamm H, Fabel H, Bartsch W. The surfactant system of the adult lung: physiology and clinical perspectives. Clin Investig 1992;70:637-57. [Crossref] [PubMed]
- Gregory TJ, Longmore WJ, Moxley MA, et al. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest 1991;88:1976-81. [Crossref] [PubMed]
- Günther A, Siebert C, Schmidt R, et al. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med 1996;153:176-84. [Crossref] [PubMed]
- Baker CS, Evans TW, Randle BJ, et al. Damage to surfactant-specific protein in acute respiratory distress syndrome. Lancet 1999;353:1232-7. [Crossref] [PubMed]
- Davidson WJ, Dorscheid D, Spragg R, et al. Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis. Crit Care 2006;10:R41. [Crossref] [PubMed]
- Bernhard W, Pynn CJ, Jaworski A, et al. Mass spectrometric analysis of surfactant metabolism in human volunteers using deuteriated choline. Am J Respir Crit Care Med 2004;170:54-8. [Crossref] [PubMed]
- Fredenburgh LE, Kraft BD, Hess DR, et al. Effects of inhaled CO administration on acute lung injury in baboons with pneumococcal pneumonia. Am J Physiol Lung Cell Mol Physiol 2015;309:L834-46. [PubMed]
- Czikora I, Alli A, Bao HF, et al. A novel tumor necrosis factor-mediated mechanism of direct epithelial sodium channel activation. Am J Respir Crit Care Med 2014;190:522-32. [Crossref] [PubMed]
- Shabbir W, Scherbaum-Hazemi P, Tzotzos S, et al. Mechanism of action of novel lung edema therapeutic AP301 by activation of the epithelial sodium channel. Mol Pharmacol 2013;84:899-910. [Crossref] [PubMed]
- Krenn K, Croize A, Klein KU, et al. Oral inhalation of AP301 peptide activates pulmonary oedema clearance: initial results from a phase IIa clinical trial in mechanically ventilated ICU patients. Eur Respir J 2014;44:1386.
- Brenner JS. Nanomedicine for the Treatment of Acute Respiratory Distress Syndrome. The 2016 ATS Bear Cage Award-winning Proposal. Ann Am Thorac Soc 2017;14:561-4. [Crossref] [PubMed]
- Brenner JS, Greineder C, Shuvaev V, et al. Endothelial nanomedicine for the treatment of pulmonary disease. Expert Opin Drug Deliv 2015;12:239-61. [Crossref] [PubMed]