Diagnosis and prognosis—review of biomarkers for mesothelioma
Malignant pleural mesothelioma (MPM) is a highly aggressive tumor arising from pleural cell lining and is associated with asbestos exposure. Between 1940 and 1979, approximately 27.5 million people in the United States were occupationally exposed to asbestos. Over the years, there has been a rising incidence of MPM, reaching approximately 3,000 cases annually. In addition, MPM has a prolonged latency period of presenting clinically 10 to 40 years after initial exposure. Most patients are diagnosed with advanced stage disease and have median survival time of less than 12 months (1). Given the increasing incidence of MPM and its lengthy latency period, there is an urgent need for earlier diagnosis and better prognostication.
Currently, the best known clinical prognostic scoring systems for MPM patients are from European Organization for Research and Treatment of Cancer (EORTC) and Cancer and Leukemia Group B (CALGB) (2,3). Specifically, they have found that poor performance status, non-epithelioid histology, male gender, anemia, thrombocytosis, leukocytosis, and elevated LDH were poor prognostic indicators in patients with MPM. Despite the utility of these scoring systems, overall survival remains dismal and there is still a need for better prognostic biomarkers. Over the past decade, advances in molecular biology have led to the identification of several biomarkers in MPM patients with potential to serve as screening tools and for early diagnosis in high-risk populations. Here, we explore the most recent and promising markers that can play a role in improving the treatment and outlook for future patients.
Soluble mesothelin-related proteins (SMRPs)
SMRPs are found in normal mesothelin cells and are over-expressed in various cancers. They are membrane-bound peptides that can be processed to yield megakaryocyte-potentiating factor (MPF) and mesothelin, which remains attached to the cell membrane via glycophosphatidylinositol linkage (4). Further studies have shown that mesothelin promotes tumor cell survival and proliferation via activation of NF-kB pathway, resulting in increase of interleukin-6 level (5). Hollevoet and colleagues have shown that as a diagnostic marker, mesothelin has high specificity of 96% but low sensitivity of only 47% (6). With regard to prognosis, the results are inconclusive. Several studies have shown no correlation between serum mesothelin level and progression-free or overall survival (7-9). On the other hand, some have shown that at cut off values of 1 and 3.5 nmol/L, SMRP levels are inversely associated with overall survival (10-13). However, in multivariate analysis limited to epithelial MPM, the prognostic impact of SMRP on overall survival was lost. This suggests that histology remains a critical determinant of prognosis. Possible explanations for the mixed results on mesothelin as a prognostic marker include small sample sizes and heterogeneous treatment among the different studies. Therefore, studies with more standardized treatments and larger numbers of patients are needed to better understand the role of SMRP as a prognostic marker.
Osteopontin
Osteopontin is an extracellular cell adhesion protein that mediates cell-matrix interaction and cell-signaling via interaction with integrin and CD44 receptors (14). Studies have shown that osteopontin is up-regulated in cells exposed to asbestos in-vitro, as well as in rat models of asbestos-induced carcinogenesis (15). A landmark study by Pass and colleagues compared 69 patients with asbestos-related non-malignant pulmonary disease with 45 patients without exposure to asbestos and 76 patients with surgically staged pleural mesothelioma (16). They found that serum osteopontin levels were significantly higher in patients with pleural mesothelioma than in those with exposure to asbestos (P<0.001). Specifically, with a cutoff value of 48.3 ng/mL, the ROC curve in the group exposed to asbestos compared with the group with mesothelioma had sensitivity of 77.6% and a specificity of 85.5%. Further subgroup analysis showed that at cutoff value of 62.4 ng/mL, ROC curve comparing patients with stage I mesothelioma and patients with exposure to asbestos showed sensitivity of 84.6% and specificity of 88.4%. Collectively, these results initially established osteopontin as a potential diagnostic marker for MPM patients. Unfortunately, results from this study led to a whirlwind of controversy as it was able to be validated in certain studies (17-19) but not in others (10,20). Some potential explanations include the different ELISA assays used for osteopontin and different control populations used, which may not be reflective of high-risk screening populations. Nevertheless, lack of validation in separate cohorts has left the value of osteopontin as a diagnostic marker in question.
Despite controversy over diagnostic value, several studies have investigated osteopontin’s potential in prognosis, demonstrating encouraging results. Cappia and colleagues studied immunohistochemical (IHC) expression of osteopontin in short-term and long-term survivors of MPM (21). At a cutoff value of 145 histologic scoring (HScore), they found osteopontin to be an independent prognostic predictor. Similarly, others showed that low baseline plasma osteopontin levels were independently associated with favorable progression-free and overall survival (7). Most recently, Pass and colleagues combined MPM plasma biomarkers with EORTC prognostic index (PI) to determine whether it will improve the risk stratification for MPM patients (22). The authors found that higher levels of osteopontin and mesothelin were individually associated with worse prognosis after adjusting for PI. Using Harrell’s C-index to formally assess the predictive ability of the biomarker, they also showed that incorporating either plasma osteopontin or mesothelin into the predictive PI model led to a statistically significant improvement in Harrell’s C-statistic. In the final prognostic model, log-osteopontin level, EORTC clinical PI and hemoglobin level remained as independently significant predictors. This further validates the role of osteopontin as a potential prognostic marker in MPM patients.
Fibulin-3
Fibullin-3 is a conserved member of the extracellular glycoprotein fibulin family encoded by the gene epidermal growth factor, containing fibulin-like extracellular matrix protein 1 (EFEMP1) (23). Fibullin-3 has been implicated in involvement with cell morphology, growth, adhesion, and motility, especially with regard to tumorigenesis (24). Previous studies have investigated fibulin-3 levels in plasma and pleural effusion, as well as fibulin-3 IHC expression in tumor tissues (25). They found that plasma fibulin-3 levels were significantly higher in patients with MPM, compared to those with only asbestos exposure. Similarly, effusion fibulin-3 levels were significantly higher in patients with MPM compared to those with pleural effusion unrelated to MPM. The authors also showed that at a cutoff value of 52.8 ng/mL, the ROC curve for plasma fibulin-3 level in patients with and without MPM had sensitivity of 96.7% and specificity of 95.5%. Collectively, these results have established fibulin-3 as a potential biomarker for patients with MPM, but it still needs to be prospectively validated. While no prospective validation studies have been done for fibulin-3, recent retrospective analysis of two cohorts of patients with MPM showed that plasma fibulin-3 level had low diagnostic accuracy as it was significantly elevated in one (Sydney cohort) but not the other (Vienna cohort) (26). Even though pleural effusion fibulin-3 level was not significantly different between cases and control groups, low levels were significantly associated with prolonged survival and therefore, independently associated with prognosis with a hazard ratio of 9.92. While fibulin-3 still holds promise as a biomarker for MPM patients, further prospective validation is needed.
High-mobility group box 1 (HMGB1)
HMGB1is a typical damage associated molecular pattern (DAMP) and a key mediator of inflammation. Recent studies have shown that asbestos exposure leads to necrosis of primary human mesothelial cells, resulting in release of HMGB-1, which binds to its main receptor and causes Nalp3 inflammasome activation and IL-1b secretion (27-29). This cascade has been linked to asbestos-related carcinogenesis. Studies have shown that higher serum HMGB1 level is found in patients with MPM compared to control group with only asbestos-exposure (no MPM) (30). Furthermore, at a cutoff value of 9 ng/mL, there is significant negative correlation between serum HMGB1 level and survival, suggesting a potential role for HMGB1 as a prognostic marker. Napolitano and colleagues have also shown that total HMGB1 level in blood was significantly higher in MPM patients and asbestos-exposed patients, when compared to healthy controls (31). Specifically, hyperacetylated HMGB1 level was significantly higher in MPM patients, compared with asbestos-exposed patients and healthy controls. At a cutoff value of 2.0 ng/mL, they found that serum hyperacetylated HMGB1 had sensitivity and specificity of 100% in differentiating MPM patients from asbestos-exposed individuals and healthy controls. These results thus suggest a role for hyperacetylated HMGB1 as a potential diagnostic marker to differentiate MPM patients.
Micro-RNA (miRNA)
miRNAs are a family of small non-coding RNAs, approximately 21–25 nt long, responsible for regulating gene expression by inhibiting translation of target messenger RNAs by pairing with messenger RNA recognition elements (32). In recent years, miRNAs from MRM cells or sera have been proposed as new biomarkers. Specifically, Bononi and colleagues analyzed circulating miRNAs from serum samples of MPM patients, asbestos-exposed workers, and healthy subjects (33). Using microarray and RT-qPCR technologies, they identified three circulating miRNAs that were upregulated in MPM patients compared to the control groups—miRNA 197-3p, miRNA-1281 and miRNA 32-3p. They further elucidated that miR-197 down-regulates the FOXO3 gene, while miR-32-3p down-regulates the tumor suppressor gene PTEN and the anti-proliferative factor BTG2, which suggest that these events may participate in MPM carcinogenesis.
Other studies have suggested miRNAs as potential prognostic markers. Pass and colleagues performed microarray analyses on 9 MPM cell lines and 129 fresh-frozen samples from resected MPM patients (34). They found that miRNA-29 expression levels were higher in patients with epithelioid histology. Furthermore, in the epithelial cohort of patients, miRNA-29 was found to be an independent prognostic factor since its higher expression was able to predict a more favorable prognosis (OS 21.6 months) as compared to low expression level (OS 9.1 months). Analysis of the entire cohort of patients, irrespective of histology, showed that miRNA-29 remained an independent predictor of survival, together with stage and lymph node involvement. Collectively, these results suggest that miRNA-29 is an independent prognostic marker for predicting time to progression and time of survival after surgery in MPM patients. More recently, Kirschner and colleagues constructed an miRNA signature from microarray profiling and differential expression of six miRNAs from patients who underwent extra-pleural pneumonectomy (35). ROC analysis of the six-miRNA signature showed a good prognostic role with AUC of 0.867 and accuracy in survival prediction of approximately 90%. When validated in separate cohort of patients who underwent palliative surgery, the ROC curve reported an accuracy of 71.9%, in which positive patients showed a differential survival benefit of 8.9 months between good and poor prognosis groups (15.4 vs. 6.5 months, respectively). These results suggest that the clinical utility of miRNAs should be further explored.
Proteomics
Slow Off-Rate Modified Aptamers (SOMAmers) are short, single stranded deoxynucleotides with ability to bind discrete molecular targets and they have been used in recent studies to develop proteomic assays (36). SOMAmers, as capture reagents, have several advantages over traditional antibody-based immunoassays, including high sensitivity and specificity, dynamic range, accurate quantification and reproducibility, and the ability of measure thousands of human proteins in small volumes of biological samples with low limits of detection (36,37). Utilizing the SOMAmer technology, Ostroff and colleagues discovered a candidate 13 biomarker panel for the detection of MPM in asbestos-exposed individuals with an AUC of 0.95 and an overall accuracy of 92% (38). The candidate biomarker panel consisted of both inflammatory and proliferative proteins, none of which had been previously associated with MPM, but both processes have been implicated in asbestos-induced carcinogenesis. Furthermore, sensitivity of the biomarker panel correlated with pathologic stages, such that 77% of stage I, 93% of stage II, 96% of stage III and 96% of stage IV cases were detected. These results provide the foundation for surveillance and early diagnosis of MPM in high-risk population.
Peripheral blood based markers
Chronic inflammation is critically involved in the pathogenesis of MPM and inflammation-based prognostic scores, such as lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), have been studied as potential prognostic markers. One retrospective review study included 150 patients with biopsy-proven MPM and found that elevated LMR was significantly associated with prolonged overall survival (39). Specifically, patients with LMR greater than 2.74 had median overall survival of 14 months compared to 5 months in patients with LMR less than 2.74. This association between LMR and overall survival was confirmed in the same study using multivariate analysis and led the authors to conclude that LMR is an independent prognostic marker for overall survival in MPM patients (39). With regard to other inflammation-based prognostic scores, such as NLR, the results are somewhat conflicting. In a cohort of consecutive, previously untreated patients diagnosed with MPM, Meniawy and colleagues found that baseline NLR greater than 5 did not predict worse overall survival (40). On multivariate analysis, age, histology, performance status, weight loss, chest pain and platelet count remained significant such that they concluded the EORTC and CALGB prognostic groups were validated as predictive of overall survival, but not NLR (40). On the other hand, there have been several studies that found baseline NLR to be an independent predictor of better survival and this has been subsequently validated in other independent studies (41-46). More recently, one study found that in a cohort of 52 patients diagnosed with MPM, patients with epithelial histology, performance status, NLR less than 5 and positive Wilms’ tumor gene (WT1) expression were significant prognostic factors for overall survival on univariate analysis (46). However, on multivariate analysis, only epithelial histology and WT1 expression remained as significant prognostic factors for overall survival (46). Possible explanations to account for the varying results from different studies on NLR include different cut-off values for NLR, heterogeneous patient populations with different treatment modalities and non-randomized allocation with its inherent biases. Therefore, further randomized, prospective validation studies are needed to better elucidate the role of NLR as a prognostic marker for MPM patients.
Conclusions
Recent years have seen a number of developments in biomarker research for malignant mesothelioma prognosis and diagnosis (Table S1). Multiple studies have shown promising results for both new and previously explored markers, with the most potential coming from those most widely investigated, such as osteopontin, SMPR, fibulin-3, HMGB1, and miRNAs. Others are beginning to be explored as well, including Neutrophil-Lymphocyte Ration (LMR), integrin, BAP-1, calretrin, caveolin-1, and P16-CDKN2A, but these will require much more work and validation in the future. However, common to many studies are limitations such as lack of standardized treatments and assays that may affect results and analysis. Furthermore, low patient numbers in certain studies limit the conclusiveness of results and suggest the need for increased cooperation among research centers in combining cohorts and increasing study sizes.
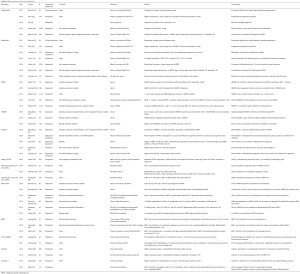
Full table
Malignant mesothelioma is an aggressive disease with diffuse nature, low median survival, and prolonged latency presenting difficulty in prognosis, diagnosis, and treatment. Incidence is increasing annually, as millions exposed to asbestos during the second half of the 20th Century are being diagnosed decades later. Thus, while progress is being made in mesothelioma biomarker discovery and investigation, much work remains to be done for improved prognosis and diagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zucali PA, Ceresoli GL, De Vincenzo F, et al. Advances in the biology of malignant pleural mesothelioma. Cancer Treat Rev 2011;37:543-58. [Crossref] [PubMed]
- Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [Crossref] [PubMed]
- Herndon JE, Green MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998;113:723-31. [Crossref] [PubMed]
- Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res 2004;10:3937-42. [Crossref] [PubMed]
- Tang Z, Qian M, Ho M. The role of mesothelin in tumor progression and targeted therapy. Anticancer Agents Med Chem 2013;13:276-80. [Crossref] [PubMed]
- Hollevoet K, Reitsma JB, Creaney J, et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: an individual patient data meta-analysis. J Clin Oncol 2012;30:1541-9. [Crossref] [PubMed]
- Hollevoet K, Nackaerts K, Gosselin R, et al. Soluble mesothelin, megakaryocyte potentiating factor, and osteopontin as markers of patient response and outcome in mesothelioma. J Thorac Oncol 2011;6:1930-7. [Crossref] [PubMed]
- Hollevoet K, Nackaerts K, Thas O, et al. The effect of clinical covariates on the diagnostic and prognostic value of soluble mesothelin and megakaryocyte potentiating factor. Chest 2012;141:477-84. [Crossref] [PubMed]
- Creaney J, Dick IM, Meniawy TM, et al. Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax 2014;69:895-902. [Crossref] [PubMed]
- Grigoriu BD, Scherpereel A, Devos P, et al. Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin Cancer Res 2007;13:2928-35. [Crossref] [PubMed]
- Cristaudo A, Foddis R, Vivaldi A, et al. Clinical significance of serum mesothelin in patients with mesothelioma and lung cancer. Clin Cancer Res 2007;13:5076-81. [Crossref] [PubMed]
- Schneider J, Hoffmann H, Dienemann H, et al. Diagnostic and prognostic value of soluble mesothelin-related proteins in patients with malignant pleural mesothelioma in comparison with benign asbestosis and lung cancer. J Thorac Oncol 2008;3:1317-24. [Crossref] [PubMed]
- Linch M, Gennatas S, Kazikin S, et al. A serum mesothelin level is a prognostic indicator for patients with malignant mesothelioma in routine clinical practice. BMC Cancer 2014;14:674. [Crossref] [PubMed]
- Wai PY, Kuo PC. The role of Osteopontin in tumor metastasis. J Surg Res 2004;121:228-41. [Crossref] [PubMed]
- Sandhu H, Dehnen W, Roller M, et al. mRNA expression patterns in different stages of asbestos-induced carcinogenesis in rats. Carcinogenesis 2000;21:1023-9. [Crossref] [PubMed]
- Pass HI, Lott D, Lonardo F, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med 2005;353:1564-73. [Crossref] [PubMed]
- Cristaudo A, Bonotti A, Simonini S, et al. Combined serum mesothelin and plasma osteopontin measurements in malignant pleural mesothelioma. J Thorac Oncol 2011;6:1587-93. [Crossref] [PubMed]
- Cristaudo A, Foddis R, Bonotti A, et al. Comparison between plasma and serum osteopontin levels: usefulness in diagnosis of epithelial malignant pleural mesothelioma. Int J Biol Markers 2010;25:164-70. [PubMed]
- Rai AJ, Flores RM, Mathew A, et al. Soluble mesothelin related peptides (SMRP) and osteopontin as protein biomarkers for malignant mesothelioma: analytical validation of ELISA based assays and characterization at mRNA and protein levels. Clin Chem Lab Med 2010;48:271-8. [Crossref] [PubMed]
- Paleari L, Rotolo N, Imperatori A, et al. Osteopontin is not a specific marker in malignant pleural mesothelioma. Int J Biol Markers 2009;24:112-7. [Crossref] [PubMed]
- Cappia S, Righi L, Mirabelli D, et al. Prognostic role of osteopontin expression in malignant pleural mesothelioma. Am J Clin Pathol 2008;130:58-64. [Crossref] [PubMed]
- Pass HI, Goparaju C, Espin-Garcia O, et al. Plasma biomarker enrichment of clinical prognostic indices in malignant pleural mesothelioma. J Thorac Oncol 2016;11:900-9. [Crossref] [PubMed]
- Zhang Y, Marmorstein LY. Focus on molecules: fibulin-3 (EFEMP1). Exp Eye Res 2010;90:374-5. [Crossref] [PubMed]
- Obaya AJ, Rua S, Moncada-Pazos A, et al. The dual role of fibulins in tumorigenesis. Cancer Lett 2012;325:132-8. [Crossref] [PubMed]
- Pass HI, Levin SM, Harbut MR, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med 2012;367:1417-27. [Crossref] [PubMed]
- Kirschner MB, Pulford E, Hoda MA, et al. Fibulin-3 levels in malignant pleural mesothelioma are associated with prognosis but not diagnosis. Br J Cancer 2015;113:963-9. [Crossref] [PubMed]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007;81:1-5. [Crossref] [PubMed]
- Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin Cancer Res 2012;18:598-604. [Crossref] [PubMed]
- Wang Y, Faux SP, Hallden G, et al. Interleukin-1beta and tumour necrosis factor-alpha promote the transformation of human immortalised mesothelial cells by erionite. Int J Oncol 2004;25:173-8. [PubMed]
- Rai AJ, Flores RM, Mathew A, et al. Soluble mesothelin related peptides (SMRP) and osteopontin as protein biomarkers for malignant mesothelioma: analytical validation of ELISA based assays and characterization at mRNA and protein levels. Clin Chem Lab Med 2010;48:271-8. [Crossref] [PubMed]
- Napolitano A, Antoine DJ, Pellegrini L, et al. HMGB1 and its hyperacetylated isoform are sensitive and specific serum biomarkers to detect asbestos exposure and to identify mesothelioma patients. Clin Cancer Res 2016;22:3087-96. [Crossref] [PubMed]
- Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol 2014;9:287-314. [Crossref] [PubMed]
- Bononi I, Comar M, Puozzo A, et al. Circulating microRNAs found dysregulated in ex-exposed asbestos workers and pleural mesothelioma patients as potential new biomarkers. Oncotarget 2016;7:82700-11. [PubMed]
- Pass HI, Goparaju C, Ivanov S, et al. hsa-miR-29c* is linked to the prognosis of malignant pleural mesothelioma. Cancer Res 2010;70:1916-24. [Crossref] [PubMed]
- Kirschner MB, Cheng YY, Armstrong NJ, et al. MiR-score: a novel 6-microRNA signature that predicts survival outcomes in patients with malignant pleural mesothelioma. Mol Oncol 2015;9:715-26. [Crossref] [PubMed]
- Kraemer S, Vaught JD, Bock C, et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: a SOMAmer-based, streamlined multiplex proteomic assay. PLoS One 2011;6:e26332. [Crossref] [PubMed]
- Kuramitsu Y, Miyamoto H, Tanaka T, et al. Proteomic differential display analysis identified upregulated astrocytic phosphoprotein PEA-15 in human malignant pleural mesothelioma cell lines. Proteomics 2009;9:5078-89. [Crossref] [PubMed]
- Ostroff RM, Mehan MR, Stewart A, et al. Early detection of malignant pleural mesothelioma in asbestos-exposed individuals with a noninvasive proteomics-based surveillance tool. PLoS One 2012;7:e46091. [Crossref] [PubMed]
- Yamagishi T, Fujimoto N, Nishi H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung Cancer 2015;90:111-7. [Crossref] [PubMed]
- Meniawy TM, Creaney J, Lake RA, et al. Existing models, but not neutrophil-to-lymphocyte ratio, are prognostic in malignant mesothelioma. Br J Cancer 2013;109:1813-20. [Crossref] [PubMed]
- Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res 2010;16:5805-13. [Crossref] [PubMed]
- Kao SC, Klebe S, Henderson DW, et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol 2011;6:1923-9. [Crossref] [PubMed]
- Cedrés S, Montero MA, Martinez P, et al. Exploratory analysis of activation of PTEN-PI3K pathway and downstream proteins in malignant pleural mesothelioma (MPM). Lung Cancer 2012;77:192-8. [Crossref] [PubMed]
- Pinato DJ, Mauri FA, Ramakrishnan R, et al. Inflammation-based prognostic indices in malignant pleural mesothelioma. J Thorac Oncol 2012;7:587-94. [Crossref] [PubMed]
- Kao SC, Vardy J, Chatfield M, et al. Validation of prognostic factors in malignant pleural mesothelioma: a retrospective analysis of data from patients seeking compensation from the New South Wales Dust Diseases Board. Clin Lung Cancer 2013;14:70-7. [Crossref] [PubMed]
- Cedrés S, Montero MA, Zamora E, et al. Expression of wilms' tumor gene (WT1) is associated with survival in malignant pleural mesothelioma. Clin Transl Oncol 2014;16:776-82. [Crossref] [PubMed]
- Bonotti A, Simonini S, Pantani E, et al. Serum mesothelin, osteopontin and vimentin: useful markers for clinical monitoring of malignant pleural mesothelioma. Int J Biol Markers 2017;32:e126-31. [Crossref] [PubMed]
- Hu ZD, Liu XF, Liu XC, et al. Diagnostic accuracy of osteopontin for malignant pleural mesothelioma: a systematic review and meta-analysis. Clin Chim Acta 2014;433:44-8. [Crossref] [PubMed]
- Felten MK, Khatab K, Knoll L, et al. Changes of mesothelin and osteopontin levels over time in formerly asbestos-exposed power industry workers. Int Arch Occup Environ Health 2014;87:195-204. [Crossref] [PubMed]
- Bayram M, Dongel I, Akbaş A, et al. Serum biomarkers in patients with mesothelioma and pleural plaques and healthy subjects exposed to naturally occurring asbestos. Lung 2014;192:197-203. [Crossref] [PubMed]
- Creaney J, Sneddon S, Dick IM, et al. Comparison of the diagnostic accuracy of the MSLN gene products, mesothelin and megakaryocyte potentiating factor, as biomarkers for mesothelioma in pleural effusions and serum. Dis Markers 2013;35:119-27. [Crossref] [PubMed]
- Demir M, Kaya H, Taylan M, et al. Evaluation of new biomarkers in the prediction of malignant mesothelioma in subjects with environmental asbestos exposure. Lung 2016;194:409-17. [Crossref] [PubMed]
- Santarelli L, Staffolani S, Strafella E, et al. Combined circulating epigenetic markers to improve mesothelin performance in the diagnosis of malignant mesothelioma. Lung Cancer 2015;90:457-64. [Crossref] [PubMed]
- Filiberti R, Marroni P, Spigno F, et al. Is soluble mesothelin-related protein an upfront predictive marker of pleural mesothelioma? A prospective study on Italian workers exposed to asbestos. Oncology 2014;86:33-43. [Crossref] [PubMed]
- Ferro P, Canessa PA, Battolla E, et al. Mesothelin is more useful in pleural effusion than in serum in the diagnosis of pleural mesothelioma. Anticancer Res 2013;33:2707-13. [PubMed]
- Filiberti R, Marroni P, Mencoboni M, et al. Individual predictors of increased serum mesothelin in asbestos-exposed workers. Med Oncol 2013;30:422. [Crossref] [PubMed]
- Tabata C, Kanemura S, Tabata R, et al. Serum HMGB1 as a diagnostic marker for malignant peritoneal mesothelioma. J Clin Gastroenterol 2013;47:684-8. [Crossref] [PubMed]
- Kaya H, Demir M, Taylan M, et al. Fibulin-3 as a diagnostic biomarker in patients with malignant mesothelioma. Asian Pac J Cancer Prev 2015;16:1403-7. [Crossref] [PubMed]
- Rapisarda V, Ledda C, Migliore M, et al. FBLN-3 as a biomarker of pleural plaques in workers occupationally exposed to carcinogenic fibers: a pilot study. Future Oncol 2015;11:35-7. [Crossref] [PubMed]
- Laszlo V, Hoda MA, Garay T, et al. Epigenetic down-regulation of integrin α7 increases migratory potential and confers poor prognosis in malignant pleural mesothelioma. J Pathol 2015;237:203-14. [Crossref] [PubMed]
- Matsumoto S, Nabeshima K, Hamasaki M, et al. Upregulation of microRNA-31 associates with a poor prognosis of malignant pleural mesothelioma with sarcomatoid component. Med Oncol 2014;31:303. [Crossref] [PubMed]
- Gayosso-Gómez LV. Identification of circulating miRNAs profiles that distinguish malignant pleural mesothelioma from lung adenocarcinoma. EXCLI J 2014;13:740-50. [PubMed]
- Andersen M, Grauslund M, Ravn J, et al. Diagnostic potential of miR-126, miR-143, miR-145, and miR-652 in malignant pleural mesothelioma. J Mol Diagn 2014;16:418-30. [Crossref] [PubMed]
- Wright CM, Kirschner MB, Cheng YY, et al. Long non coding RNAs (lncRNAs) are dysregulated in Malignant Pleural Mesothelioma (MPM). PLoS One 2013;8:e70940. [Crossref] [PubMed]
- Hwang HC, Pyott S, Rodriguez S, et al. BAP1 immunohistochemistry and p16 FISH in the diagnosis of sarcomatous and desmoplastic mesotheliomas. Am J Surg Pathol 2016;40:714-8. [Crossref] [PubMed]
- Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 2015;28:1043-57. [Crossref] [PubMed]
- Farzin M, Toon CW, Clarkson A, et al. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology 2015;47:302-7. [Crossref] [PubMed]
- Hwang H, Tse C, Rodriguez S, et al. p16 FISH deletion in surface epithelial mesothelial proliferations is predictive of underlying invasive mesothelioma. Am J Surg Pathol 2014;38:681-8. [Crossref] [PubMed]
- Thapa B, Walkiewicz M, Murone C, et al. Calretinin but not caveolin-1 correlates with tumour histology and survival in malignant mesothelioma. Pathology 2016;48:660-5. [Crossref] [PubMed]
- Righi L, Cavallo MC, Gatti G, et al. Tumor/stromal caveolin-1 expression patterns in pleural mesothelioma define a subgroup of the epithelial histotype with poorer prognosis. Am J Clin Pathol 2014;141:816-27. [Crossref] [PubMed]


