Volumetric assessment in malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) has a complex morphology, often compared to the rind of an orange, but confined to the ipsilateral hemithorax in early stages, with a propensity to invade multiple planes simultaneously as the disease progresses. Combined with poor separation from adjacent tissues by all imaging modalities, radiographic assessment is challenging and does not correlate with pathology (1). Multimodality imaging approach is generally employed to assess resectability (2), in most clinical practices the radiologists will not attempt clinical staging, rather providing a descriptive report and often letting the surgeons and oncologists to use the information to derive a clinical stage prior to treatment stratification (3).
Radiologic assessment
The imaging appearance of MPM can be nonspecific, ranging from pleural effusion and circumferential rind like pleural thickening, to pleural masses (2). CT is the most commonly used imaging modality for diagnosis, staging and assessing response to therapy. Unilateral pleural effusion (74%), circumferential, nodular pleural thickening (92%), and thickening of the interlobular septa (86%) are the most common CT manifestations (2,4). In the majority of cases the pleural thickening develops first along the diaphragmatic surface of pleura and continues in a circumferential manner, extending towards the apex; in some cases there may be localized pleural masses. This manner of growth puts several structures at risk of invasion as the tumor progresses. Even though the overall disease burden is confined to the involved hemithorax in early stages, with the passage of time, direct invasion of the chest wall, mediastinum, pericardium and diaphragm can occur, and metastases to the lymph nodes, bones, lungs, or distant sites can be seen at time of presentation (5-7).
MRI is superior to CT in detecting occult chest wall, involvement of bone, interlobar fissures, diaphragm (particularly transmural involvement and extension through the diaphragm), and endothoracic fascia (6,8-10). Recent advances in MR techniques such as Diffusion Weighted MRI and Dynamic Contrast enhanced MRI can be used to assess histological subtypes and also to assess response to therapy (8,11,12). However, MRI has limited utility in settings where surgical management is not under consideration. The main strength of FDG-PET-CT is in its ability to detect distal and occult metastatic lesions that would not be apparent by other modalities (13), therefore is not a cost effective strategy in early stage MPM patients. The metabolic activity of the tumor has been shown to portend poorer prognosis with shorter survival, with an association between higher maximal standardized uptake value (SUVmax) and increased aggressiveness of the tumor (13,14). However PET-CT does not contribute substantially in the improvement in clinical staging (15), but can be helpful in assessing response to therapy. The accuracy of assessing nodal involvement by imaging continues to be problematic, with an inability to predict involvement accurately, even by even a multimodality approach. Additionally there is very limited value added by FDGPET in patients who have undergone prior talc or chemical pleurodesis, as inflammatory response can cause increased avidity in the pleura for prolonged periods of time and can also result in increase in size of the mediastinal and hilar nodes with increased SUV uptake (14,16).
Clinical staging of MPM using current imaging modalities does not accurately predict either pathologic stage or prognosis and is plagued by high interobserver variability (17-19). This is not surprising since the pathologic classification is based on microscopic assessment of tumor invasion at a cellular scale that is well beyond the spatial resolution of any combined multi-modality approach. Combined with the lack of structured radiographic reporting and qualitative or descriptive categorization of invasion of structures by the tumor, there can be high degree of variability in reporting, even among experienced radiologists (20). Therefore, there is a greater emphasis on determining resectability rather than an attempt at clinical staging in these patients. If deemed unresectable, serial imaging with CT or PETCT to determine response to therapy by comparing differences in uptake and tumor measurements is often done (21-25).
Determination of resectability is highly variable among institutions and is often considered on a case-by-case basis based on imaging appearance, performance status, and individual surgical practice. Features suggestive of un-resectability include (I) extensive or diffuse chest wall invasion; (II) direct mediastinal invasion, or invasion of vascular structures; (III) mediastinal lymph nodal involvement; and (IV) direct intra-abdominal extension or distant metastatic disease (2,4).
Due to these difficulties with clinical staging, quantitative tumor measurements have been proposed. The International Association for the Study of Lung Cancer (IASLC)/International Mesothelioma Interest Group (IMIG) database collected measurements of pleural thickness on CT and correlated them with outcomes; they found that maximal pleural thickness measured on axial CT correlated with T stage (TNM 7th edition), overall stage, nodal stage and survival (17). A maximal pleural thickness greater than 5.1 mm was associated with a median survival of 18 months, with a median survival of 24 months in those with a pleural thickness less than 5.1 mm (17).
Quantitative assessment of overall tumor burden can be used for determining treatment response. One-dimensional, bi-dimensional (26) and mesothelioma specific modified Response Evaluation of Solid Tumor criteria (RECIST) (21) have been proposed as potential quantitative methods of MPM tumor measurement in the evaluation of treatment response. These methods are based on the measurement of maximal tumor thickness in one or more anatomical planes. Due to the aforementioned difficulties in measuring MPM these methods have found to be unreliable, with high inter- and intra-observer variation when compared with volumetric analysis (19).
Alternative staging strategies and prognostic models
Clinical Staging for MPM has not been considered useful as it has for other malignancies to determine prognosis or inform treatment decisions, which has led to the development and validation of other prognostic factors derived from clinical and pathological factors (27-31). The European Organization for Research and Treatment of Cancer (EORTC) (32) and the Cancer and Leukemia Group B (CALGB) (33) have identified several prognostic factors from pooled cohorts of patients treated on chemotherapy trials prospectively; worse prognosis was associated with poor performance status, non-epithelial histology, advanced stage (stage IV), gender, age and anemia, along with metabolic activity and tumor volume (22,30,34,35).
Measurement of tumor volume on CT
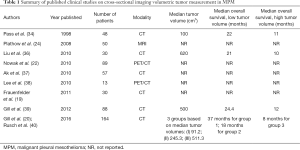
Tumor volume measurement on cross-sectional imaging has been proposed as a quantitative measurement with prognostic significance (Table 1). Pass et al. provided the seminal work on volumetric tumor burden in MPM in 1998. They assessed a number of potential prognostic markers in 95 patients undergoing surgery for MPM including tumor volume derived from CT scans, and concluded that a tumor volume greater than 100 cm3 was associated with decreased survival (34). They measured preoperative tumor volume from CT scans of patients with MPM undergoing extrapleural pneumonectomy (EPP), finding a significantly improved median survival of 22 months in patients with a preoperative tumor volume of less than 100 cm3, compared with 11 months in those with a tumor volume greater than 100 cm3. Tumor volume was significantly smaller (median tumor volume 50 cm3) in patients with node negative disease compared with those with nodal metastasis (median tumor volume 166 cm3) (34). The largest single center study of CT-derived volume by Gill et al. assessed tumor volume in 88 patients with epithelioid subtype MPM who underwent EPP, finding a significantly improved median survival in patients with a preoperative volume of less than 500 cm3 (24 months) as compared with those greater than 500 cm3 (12 months) (39).
Full table
The original method for tumor volume measurement on CT described by Pass et al. required the radiologist to manually outline tumor borders on workstation on each slice, with the sum of the measurements then used to calculate overall tumor volume (34). This was a painstakingly slow technique requiring considerable manual labor, and at that time was too cumbersome to be included in the routine clinical workflow. Ak et al. measured tumor volume with a method based on the Cavalieri principle of stereology, involving counting equidistant points superimposed on the CT images and deriving a volume based on the number of points occupied by tumor (37). This method, again, was not practical and could not be incorporated in the routine clinical workflow. Advances in technology, the increasing need for quantitative metrics in clinical trials and the availability of hybrid workstations have led to the advent of less labor-intensive, semiquantitative methods of tumor volume measurement (41). These voxel-based semiquantitative methods have subsequently been used in validation (42) and clinical studies, and are most readily applicable to everyday clinical radiological practice.
Proprietary software allowing semiautomatic voxel-based three-dimensional tumor segmentation is now readily available, and was used in the recent multicenter study on CT-derived tumor volume (Vitrea Enterprise, Vital images, Minnetonka, MN, USA) (20,40). This type of software first automatically segments the tumor volume based on adjustable Hounsfield unit (HU) values (20–80 HU), followed by manual correction of the tumor contours by the radiologist. This process allows a rapid measurement of tumor volume that can be applied to routine clinical reporting.
To ensure accurate volume measurement, tumor needs to be accurately delineated from adjacent structures, such as atelectasis lung, pleural fluid, chest wall musculature and lymph nodes. Lack of intravenous (IV) contrast, prior pleurodesis and poor signal-to-noise ratio are the most common causes cited of erroneous volume measurements (20). CT with IV contrast is preferable, with delayed phase acquired approximately 120 seconds post IV contrast injection preferred as the optimal scan timing for distinguishing tumor from adjacent structures (20). Volumetric quantification is ideally performed on thin-section CT reconstructions, which are readily available with modern CT technology. A slice thickness and interval of 1mm provides an isotropic dataset that is ideal for three-dimensional tumor segmentation. Thicker section reconstructions, such as a slice thickness of 3 or 5mm have been used, but are more challenging to segment, and may provide less accurate volume measurements (20,41,43).
In the last decade several quantitative strategies to assess response and stratify prognosis in MPM have been evaluated. Tumor volume measurements from cross sectional imaging have been compared to other quantitative methods of therapy response measurement in MPM. Plathow et al. measured MPM tumor volume on 50 patients and compared it to RECIST and modified RECIST in patients undergoing chemotherapy, finding that tumor volume measured on MRI is a good prognostic indicator and metric of treatment response or failure (24). Tumor volume measurement on CT has similarly been found to be useful in assessing treatment response. Liu et al. assessed treatment response in patients with MPM by measuring tumor volume on CT before and after therapy, finding that tumor volume reduction post treatment is significantly associated with improved survival, and that patients with a tumor volume of less than 620 cm3 at diagnosis do better than those with higher volumes, with median survivals of 21 and 10 months respectively (36). Ak et al. demonstrated similar results (37), Frauenfelder et al. found that tumor volume measurement on CT is a more reliable measure of chemotherapy response and predictor of outcomes in MPM than RECIST (19).
The potential superior prognostic ability of tumor volume compared to clinical T stage led to the establishment of the North American Multicenter Volumetric CT Study for Clinical Staging of Malignant Pleural Mesothelioma. This was a prospective, multi-institutional feasibility study on the use of tumor volume derived from CT as a clinical T stage in patients with newly diagnosed MPM. Tumor volumes and clinical TNM staging were performed on CT scans from 130 patients from the IASLC/IMIG MPM database, with the scans analyzed on two separate sites. The authors found poor correlation of T staging between the two reporting radiologists, but good correlation for tumor volume measurements. Tumor volume correlated significantly with pathological T stage, pathological N stage, as well as with overall survival. The study group found that dividing the patients into three groups, best defined by average tumor volumes of 91, 245 and 511 cm3 respectively had prognostic significance superior to traditional clinical T staging, with median survival of 37, 18 and 8 months respectively for the three groupings (40). The finding that patients with a tumor volume of greater than 500 cm3 have a worse overall survival is in line with previous single-institution studies on tumor volumes in MPM (34,39). It was not possible to discern a clear link between tumor volume and histological subtype, likely due to the preponderance of epithelioid disease (81% cases) in their cohort.
CT remains the most widely studied imaging modality for MPM volume assessment. It is less costly, and more widely available than MRI or PET/CT, and will likely remain the central imaging modality for the diagnosis and staging of MPM.
Measurement of tumor volume on MRI and PET/CT
Other modalities, such as MRI (24) and 18F-FDG PET/CT (22,36,44) have been successfully used to measure tumor volume in MPM (Table 1). For MRI, tumor segmentation has been performed using a HASTE (half-Fourier single-shot fast spin-echo) sequence, but this can be limited by signal inhomogeneity and motion artifacts, with a segmentation time of 10 minutes described per patient (45). Tumor segmentation on PET/CT allows calculation of the volume of metabolically active tumor by segmenting the volume of interest using an adaptive threshold based on SUV (22,23,38,44). The total glycolytic volume (TGV) is a measure of tumor volume and metabolic activity, with a correlation between TGV and tumor activity (46). Measurement of the volume of metabolically active tumor on 18F-FDG PET/CT was incorporated into a prognostic model, incorporating both tumor volume and glycolysis, termed TGV (22,38). SUV alone did not significantly predict survival, suggesting that tumor volume provides the main prognostic information (22).
Lung volume measurement
Until recently, calculation of overall tumor volume for patients with MPM were time prohibitive, and single dimension or bi-dimensional linear measurements continue to be the standard of care for response assessment (21,47). Even though tumor volume calculation is becoming increasingly common, it is still somewhat labor intensive needing significant manual correction even with the state of art software packages. Labby et al. found that lung volume along with tumor volume were also predictor of survival (48). Computing lung volumes is relatively easier than computing tumor volume and if the circumferential rind was to decrease in volume the lung expansion would increase thus could potentially serve as a surrogate of response.
Furthermore normalizing the ipsilateral lung volume by the contralateral lung volumes could correct for differences in respiratory phase between a patient’s CT scans, and changes in normalized lung volume could potentially form a useful response assessment metric (48). Even though this strategy could work in the non-surgical patients, application to the surgical cohort could be problematic. Variable amounts of lung may be resected during pleurectomy, and resection of the phrenic nerve could lead to diaphragmatic paralysis, thus affecting lung re-expansion and could affect the accuracy and reproducibility of the measurement.
Pathological tumor volume
Kircheva et al. measured tumor weight and volume by weighing pathologically resected specimens and displacing water in 116 patients with MPM who had undergone EPP (49). The authors found that patients with a median tumor volume of greater than 567 cm3 had a median survival of 11.8 months, compared with 22.6 months for patients with a median tumor volume of less than 567 cm3, and that tumor volume is a better predictor of survival than clinical T stage. Even though pathological tumor volume thresholds were similar to those derived from preoperative CT scans, depending on the type of surgery and amount of resection of adjacent tissue during surgery along with the tumor it may not be a reproducible strategy. Tumor volume could also be difficult to assess in cases in which patients underwent extrapleural pneumonectomy or palliative resection.
Translation of tumor volume measurement into clinical practice
The transfer of quantitative MPM tumor volume assessment from the research field to the reading room will require education of reporting radiologists and improving their ability to use the quantification tools included into most modern picture archiving and communication (PACS) workstations. To this end, the IASLC/IMIG have proposed the creation of a training set based on their pilot cases to educate radiologists on how to perform tumor volumetry (20). Advances in imaging post-processing software will hopefully make the process of tumor volume measurement quicker and more user friendly, facilitating its incorporation as a quantitative clinical staging category for this morphologically complex tumor.
Summary
Tumor volume measurement is promising, it allows for a quantitative assessment of MPM tumor burden and has been shown to have prognostic significance. It can serve as a quantitative measure of therapeutic response in prospective clinical trials, comparing it with the standard of care strategies will allow further refining of the cut offs for response categories. Tumor volumes of greater than 500 cm3 have consistently been shown to adversely impact overall survival but further validation of the role of tumor volume in clinical staging is required with large international studies before it can be incorporated into the clinical staging algorithm. Standardization of imaging and reporting protocols across centers would allow pooling of data in this rare disease and allow development of standardization metrics, which in turn can transition tumor volume into clinical practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ripley BA, Gill RR. Chapter 16: Imaging Evaluation in Malignant Pleural Mesothelioma. In: Mineo TC. editor. Malignant Pleural Mesothelioma: Present States and Future Directions. Sharjah: Bentham Science Publishers, 2016:213-6.
- Gill RR, Gerbaudo VH, Sugarbaker DJ, et al. Current Trends in Radiologic Management of Malignant Pleural Mesothelioma. Semin Thorac Cardiovasc Surg 2009;21:111-20. [Crossref] [PubMed]
- Glastonbury CM, Bhosale PR, Choyke PL, et al. Do radiologists have stage fright? Tumor staging and how we can add value to the care of patients with cancer. Radiology 2016;278:11-2. [Crossref] [PubMed]
- Gill RR. Imaging of mesothelioma. Recent Results Cancer Res 2011;189:27-43. [Crossref] [PubMed]
- Heelan R. Staging and response to therapy of malignant pleural mesothelioma. Lung Cancer 2004;45 Suppl 1:S59-61. [Crossref] [PubMed]
- Wang ZJ, Reddy GP, Gotway MB, et al. Malignant pleural mesothelioma: evaluation with CT, MR imaging, and PET. Radiographics 2004;24:105-19. [Crossref] [PubMed]
- Marom EM, Erasmus JJ, Pass HI, et al. The role of imaging in malignant pleural mesothelioma. Semin Oncol 2002;29:26-35. [Crossref] [PubMed]
- Gill RR, Gerbaudo VH, Jacobson FL, et al. MR imaging of benign and malignant pleural disease. Magn Reson Imaging Clin N Am 2008;16:319-39. x. [Crossref] [PubMed]
- Chen W, Chen L, Yang S, et al. A novel technique for localization of small pulmonary nodules. Chest 2007;131:1526-31. [Crossref] [PubMed]
- Yamamuro M, Gerbaudo VH, Gill RR, et al. Morphologic and functional imaging of malignant pleural mesothelioma. Eur J Radiol 2007;64:356-66. [Crossref] [PubMed]
- Coolen J, De Keyzer F, Nafteux P, et al. Malignant Pleural Disease: Diagnosis by Using Diffusion-weighted and Dynamic Contrast-enhanced MR Imaging--Initial Experience. Radiology 2012;263:884-92. [Crossref] [PubMed]
- Gill RR, Umeoka S, Mamata H, et al. Diffusion-weighted MRI of malignant pleural mesothelioma: preliminary assessment of apparent diffusion coefficient in histologic subtypes. AJR Am J Roentgenol 2010;195:W125-30. [Crossref] [PubMed]
- Gerbaudo VH, Katz SI, Nowak AK, et al. Multimodality imaging review of malignant pleural mesothelioma diagnosis and staging. PET Clinics 2011;6:275-97. [Crossref] [PubMed]
- Gerbaudo VH, Britz-Cunningham S, Sugarbaker DJ, et al. Metabolic significance of the pattern, intensity and kinetics of 18F-FDG uptake in malignant pleural mesothelioma. Thorax 2003;58:1077-82. [Crossref] [PubMed]
- Nowak AK, Francis RJ, Katz SI, et al. A multimodality imaging review of malignant pleural mesothelioma response assessment. PET Clinics 2011;6:299-311. [Crossref] [PubMed]
- Erasmus JJ, Truong MT, Smythe WR, et al. Integrated computed tomography-positron emission tomography in patients with potentially resectable malignant pleural mesothelioma: Staging implications. J Thorac Cardiovasc Surg 2005;129:1364-70. [Crossref] [PubMed]
- Nowak AK, Chansky K, Rice DC, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2089-99.
- Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012;7:1631-9. [Crossref] [PubMed]
- Frauenfelder T, Tutic M, Weder W, et al. Volumetry: An alternative to assess therapy response for malignant pleural mesothelioma? Eur Respir J 2011;38:162-8. [Crossref] [PubMed]
- Gill RR, Naidich DP, Mitchell A, et al. North American Multicenter Volumetric CT Study for Clinical Staging of Malignant Pleural Mesothelioma: Feasibility and Logistics of Setting Up a Quantitative Imaging Study. J Thorac Oncol 2016;11:1335-44. [Crossref] [PubMed]
- Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004;15:257-60. [Crossref] [PubMed]
- Nowak AK, Francis RJ, Phillips MJ, et al. A novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin Cancer Res 2010;16:2409-17. [Crossref] [PubMed]
- Francis RJ, Byrne MJ, van der Schaaf AA, et al. Early prediction of response to chemotherapy and survival in malignant pleural mesothelioma using a novel semiautomated 3-dimensional volume-based analysis of serial 18F-FDG PET scans. J Nucl Med 2007;48:1449-58. [Crossref] [PubMed]
- Plathow C, Klopp M, Thieke C, et al. Therapy response in malignant pleural mesothelioma-role of MRI using RECIST, modified RECIST and volumetric approaches in comparison with CT. Eur Radiol 2008;18:1635-43. [Crossref] [PubMed]
- Veit-Haibach P, Schaefer NG, Steinert HC, et al. Combined FDG-PET/CT in response evaluation of malignant pleural mesothelioma. Lung Cancer 2010;67:311-7. [Crossref] [PubMed]
- Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981;47:207-14. [Crossref] [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]
- Chailleux E, Dabouis G, Pioche D, et al. Prognostic factors in diffuse malignant pleural mesothelioma. A study of 167 patients. Chest 1988;93:159-62. [Crossref] [PubMed]
- Nojiri S, Gemba K, Aoe K, et al. Survival and prognostic factors in malignant pleural mesothelioma: A retrospective study of 314 patients in the west part of Japan. Jpn J Clin Oncol 2011;41:32-9. [Crossref] [PubMed]
- Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 1999;68:1799-804. [Crossref] [PubMed]
- Curran D, Sahmoud T, Therasse P, van Meerbeeck J, Postmus PE, Giaccone G, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [Crossref] [PubMed]
- Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [Crossref] [PubMed]
- Herndon JE, Green MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998;113:723-31. [Crossref] [PubMed]
- Pass HI, Temeck BK, Kranda K, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1998;115:310-7-8.
- Sugarbaker DJ, Strauss GM, Lynch TJ, et al. Node status has prognostic significance in the multimodality therapy of diffuse, malignant mesothelioma. J Clin Oncol 1993;11:1172-8. [Crossref] [PubMed]
- Liu F, Zhao B, Krug LM, et al. Assessment of therapy responses and prediction of survival in malignant pleural mesothelioma through computer-aided volumetric measurement on computed tomography scans. J Thorac Oncol 2010;5:879-84. [Crossref] [PubMed]
- Ak G, Metintas M, Metintas S, et al. Three-dimensional evaluation of chemotherapy response in malignant pleural mesothelioma. Eur J Radiol 2010;74:130-5. [Crossref] [PubMed]
- Lee HY, Hyun SH, Lee KS, et al. Volume-based parameter of (18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol 2010;17:2787-94. [Crossref] [PubMed]
- Gill RR, Richards WG, Yeap BY, et al. Epithelial malignant pleural mesothelioma after extrapleural pneumonectomy: stratification of survival with CT-derived tumor volume. Ajr Am J Roentgenol 2012;198:359-63. [Crossref] [PubMed]
- Rusch VW, Gill R, Mitchell A, et al. A Multicenter Study of Volumetric Computed Tomography for Staging Malignant Pleural Mesothelioma. Ann Thorac Surg 2016;102:1059-66. [Crossref] [PubMed]
- Armato SG, Ogarek JL, Starkey A, et al. Variability in mesothelioma tumor response classification. AJR Am J Roentgenol 2006;186:1000-6. [Crossref] [PubMed]
- Sensakovic WF, Armato SG, Straus C, et al. Computerized segmentation and measurement of malignant pleural mesothelioma. Med Phys 2011;38:238-44. [Crossref] [PubMed]
- Winer-Muram HT, Jennings SG, Meyer CA, et al. Effect of varying CT section width on volumetric measurement of lung tumors and application of compensatory equations. Radiology 2003;229:184-94. [Crossref] [PubMed]
- Mamede M, Abreu-E-Lima P, Oliva MR, et al. FDG-PET/CT tumor segmentation-derived indices of metabolic activity to assess response to neoadjuvant therapy and progression-free survival in esophageal cancer: correlation with histopathology results. Am J Clin Oncol 2007;30:377-88. [Crossref] [PubMed]
- Plathow C, Staab A, Schmaehl A, et al. Computed tomography, positron emission tomography, positron emission tomography/computed tomography, and magnetic resonance imaging for staging of limited pleural mesothelioma: initial results. Invest Radiol 2008;43:737-44. [Crossref] [PubMed]
- Boucek JA, Francis RJ, Jones CG, et al. Assessment of tumour response with (18)F-fluorodeoxyglucose positron emission tomography using three-dimensional measures compared to SUVmax--a phantom study. Phys Med Biol 2008;53:4213-30. [Crossref] [PubMed]
- Armato SG 3rd, Oxnard GR, MacMahon H, et al. Measurement of mesothelioma on thoracic CT scans: a comparison of manual and computer-assisted techniques. Med Phys 2004;31:1105-15. [Crossref] [PubMed]
- Labby ZE, Armato SG, Dignam JJ, et al. Lung volume measurements as a surrogate marker for patient response in malignant pleural mesothelioma. J Thorac Oncol 2013;8:478-86. [Crossref] [PubMed]
- Kircheva DY, Husain AN, Watson S, et al. Specimen weight and volume: important predictors of survival in malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;49:1642-7. [Crossref] [PubMed]


