Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease
Introduction
The French surgeon Theodore Tuffier was probably the first one to hypothesize the existence of a relationship of type 2 diabetes mellitus (T2DM) with cancer risk in the second half of nineteenth century (1,2). He observed that patients with established T2DM exhibited a greater risk of certain cancers than those without diabetes (1,2). Consequently, he formulated the following key questions: (I) could diabetes affect the incidence of cancer? (II) could diabetes influence the natural history of cancer? and (III) could cancer affect the natural history of diabetes? (1,2).
To date, seeing that the incidence of both T2DM and cancer are rapidly increasing worldwide, the above-mentioned questions remain of considerable clinical importance for investigating the epidemiological and pathophysiological associations between T2DM and cancer risk (3-6). These questions are particularly important for the relationship between T2DM and the risk of hepatocellular carcinoma (HCC), because the incidence of this cancer has rapidly increased over the last decade and it represents now the third cause of cancer-related mortality worldwide (7,8). Moreover, there is now clear evidence that T2DM and HCC are closely linked, owing to their association with obesity, impaired insulin sensitivity and nonalcoholic fatty liver disease (NAFLD) (9-14). In particular, the increasing incidence of HCC is, in large part, attributable to the rising prevalence and incidence of NAFLD worldwide. Indeed, the prognostic impact of NAFLD per se on HCC risk is currently taking increasingly greater clinical importance. NAFLD encompasses a spectrum of progressive hepatic disease (ranging from pure steatosis to steatohepatitis [NASH] and cirrhosis) that is often observed in patients with T2DM (occurring in up to 70% of these patients) (15-19). Over the last decade, strong evidence demonstrated that patients with T2DM are more likely to develop the more severe histologic forms of NAFLD, including cirrhosis and HCC (15-19).
Given the sharp rise in the global incidence of T2DM, NAFLD and HCC, it is therefore unsurprising that in clinical practice, diabetologists often have to manage diabetes in patients who are being treated for HCC and oncologists/gastroenterologists are increasingly required to plan cancer treatment for patients with pre-existing diabetes. It is hence very important that diabetologists, oncologists, gastroenterologists but also general practitioners are aware of the strong relationship between T2DM and the risk of incident HCC.
In this clinical review, we will examine the evidence for a link between T2DM, NAFLD and HCC from an epidemiological and a pathophysiological perspective. Moreover, we will also briefly discuss the potential effect of certain hypoglycemic agents on HCC risk.
Epidemiological studies linking T2DM to cancer risk
Currently, the estimated global prevalence of T2DM is approximately 9% worldwide, with a worrying tendency to increase sharply in the next years (3,4,20,21). In parallel, the total number of deaths attributed to cancer is estimated to rise over time, mainly in developing countries (5). For instance, it was calculated that there were approximately 8 million cancer-related deaths worldwide in 2012 (5). There is also strong evidence indicating that cancer risk and mortality are progressively increasing with the rise of T2DM incidence (22-24). For example, on the basis of a pooled analysis including 97 prospective cohort studies with a total of nearly 800,000 individuals, the Emerging Risk Factors Collaboration group investigators showed that individuals with established diabetes showed a remarkably greater risk of mortality from cancer than those without diabetes (25). Interestingly, the observed relationships between the presence of diabetes and the risk of site-specific cancer deaths were strongest for the risk of liver and pancreatic cancers, intermediate for ovarian and colorectal cancers, and lowest for lung and breast cancers (25). Death from site-specific cancers was also examined in a cohort of nearly 7,200 T2DM patients from Verona (Italy) over a 10-year follow-up by reviewing death certificates (26). The authors found that mortality rates from site-specific cancers were significantly higher in T2DM patients than in the general population (26). In particular, increased risk of death from liver cancer [standard mortality ratio (SMR) =1.86, 95% confidence interval (CI): 1.44–2.38] was observed in both sexes. In addition, women with diabetes also showed higher risk of death from both pancreatic (SMR =1.78, 95% CI: 1.13–2.67) and breast cancers (SMR =1.40, 95% CI: 1.06–1.81). Other investigators subsequently reported similar findings in other countries (27-30). On the basis of all these observations, a 2010 consensus statement of experts assembled jointly by the American Diabetes Association (ADA) and the American Cancer Society (ACS) concluded that the incidence rates of liver, pancreatic, colorectal, breast, endometrial and bladder cancers were increased among individuals with T2DM (31). However, it is important to note that while some methodological considerations preclude a definitive association between T2DM and risk of some cancers, the increased HCC risk observed in patients with T2DM remains statistically significant even after adjustment for detection bias and reverse causation, so indicating that such association is clinically reliable and robust (1,26,27,32,33).
Epidemiological studies linking T2DM to HCC risk
HCC represents the commonest form of primary liver cancer (5,6). The worldwide number of incident cases of HCC has been estimated to be approximately 600,000 in 2002, 80% of which were recorded in developing countries. Table S1 summarizes the principal observational studies and meta-analyses examining the relationship between pre-existing T2DM and the risk of HCC. An early description of the existence of an association between T2DM and HCC has been reported approximately 30 years ago (34). In 1986, in a case-control study involving 105 HCC patients and 105 persons with either colorectal cancer or femoral bone fractures who were matched by age and sex, Lawson et al. firstly documented that there was an approximately four-fold risk of prevalent T2DM among patients with HCC compared with those with colorectal cancer or femoral bone fractures, irrespective of chronic viral hepatitis, alcoholic cirrhosis and hemochromatosis (34). Subsequently, in a systematic analysis based on 242 cases of primary liver cancers and 1,169 controls recorded between 1984 and 1989, La Vecchia et al. found that patients with T2DM had an approximately 2.5-fold increased risk of developing liver cancers (mainly owing to HCC), independent of metabolic factors and other potential confounding variables (35). Notably, such increased risk of HCC was also observed in patients with a diagnosis of T2DM that occurred 5 or more years before the diagnosis of HCC (35). After these pioneering reports, several other case-control and prospective studies as well as some meta-analyses have been conducted, all confirming the existence of a strong relationship between T2DM and HCC risk, irrespective of several potential confounders, including overweight/obesity, excessive alcohol consumption and chronic viral hepatitis (36-39). For example, in a large meta-analysis involving approximately 3 million people, El-Serag et al. showed that among 13 case-control studies, pre-existing diabetes was associated with risk of prevalent HCC in 9 studies (odds ratio 2.50; 95% CI: 1.8–3.5). Among 13 cohort studies, diabetes was associated with risk of incident HCC in 7 studies (hazard risk 2.50; 95% CI: 1.9–3.2). The findings were consistent in different populations, different geographic locations, and a variety of control groups. The relationship between diabetes and HCC risk remained statistically significant after adjusting for alcohol use or chronic viral hepatitis in the 10 studies that examined these risk factors (40). In another meta-analysis published 6 years later, involving 49 observational studies (32 cohort studies and 17 case-control studies), Wang et al. confirmed that pre-existing T2DM was strongly associated with increased HCC prevalence and mortality, independent of several confounding factors and metabolic variables (41). In 2013, in a large cohort study of 363,426 non-diabetic individuals and 8,588 T2DM patients without any cancer or metastasis at baseline, Schlesinger et al. reported that T2DM was associated with an increased incidence of both HCC and bile tract cancer over a follow-up of 8 years, independent of age, sex, body mass index (BMI), waist-to-height ratio, center, education level, smoking and alcohol consumption (42). In a community-based cohort study of 63,257 Chinese individuals followed-up for 10 years, Koh et al. showed that T2DM was associated with increased incidence of non-viral HCC, irrespective of age, sex, BMI, recruitment year, dialect group, education level, smoking and consumption of alcohol, coffee or tea (43). In 2015, in an Italian hospital-based case-control study of 224 HCC patients and 389 control subjects, Miele et al. found that the risk of HCC was significantly increased among patients with T2DM, especially among those with a longer duration of disease (44). Recently, in a meta-analysis of 9 studies (7 cohort studies and 2 case-control studies), Dyal et al. examined the inter-relationships of T2DM, obesity and hepatic steatosis with incidence rates of HCC in patients with chronic hepatitis C virus (HCV) (45). These authors documented that T2DM was closely associated with increased risk of HCC in this patient population, independent of age, sex, obesity, hypertension, smoking, alcohol intake, serum liver enzymes, albumin, lipids, platelet count, and presence of cirrhosis and hepatic steatosis (45). Notably, the presence of obesity and hepatic steatosis (along with T2DM) were also found to be independent predictors of incident HCC (45). These findings are clinically relevant, because they would suggest a potential synergistic, additive interaction between T2DM and other major metabolic risk factor for HCC.
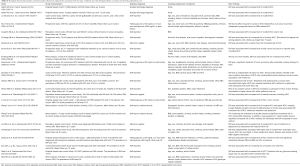
Full table
Epidemiological studies linking NAFLD to HCC risk
Over the last years, it has become increasing clear that NAFLD is strongly associated with increased risk of incident HCC. For instance, in a US population-based cohort study of 4,406 HCC cases followed-up for approximately 6 years, Sanyal et al. reported that the most common risk factor for HCC was NAFLD, followed by T2DM and HCV chronic infection (46). Almost identical findings were found in a smaller study showing that NAFLD was the most common etiology for HCC, thus outstripping chronic viral hepatitis and alcoholic liver disease (47).
Although there is now epidemiologic evidence to indicate a close relationship between NAFLD or NASH and the HCC risk, this risk appears to be particularly elevated in patients with cirrhosis, which is a well-established risk factor for HCC (11,12,48). In a national cohort of 1,500 patients who developed HCC from 2005 through 2010 from Veterans Administration hospitals, Mittal et al. showed that NAFLD was the third most common risk factor for HCC, and that more than half of these patients had cirrhosis (49). In a large meta-analysis of 17 cohort studies, 18 case-control or cross-sectional studies and 26 case-series, White et al. reported that the cohorts of NAFLD or NASH patients with few or no cases of cirrhosis showed a minimal risk of HCC (HCC mortality of 0–3% for study follow-ups up to 20 years). The cohorts of patients with NASH and cirrhosis had a substantially greater risk (HCC incidence ranging from 2.4% over 7 years to 12.8% over 3 years). However, the HCC risk was lower in the cohorts of patients with NASH-cirrhosis than for cohorts with HCV-related cirrhosis (50). Similarly, in a prospective study enrolling 315 patients with HCV-cirrhosis and 195 patients with NASH-cirrhosis who were followed-up for approximately 4 years, Ascha et al. found that the yearly cumulative HCC incidence among patients with NASH-cirrhosis was 2.6% compared to 4.0% among those with HCV-cirrhosis (51). It is important to remark that in all the above-mentioned studies other major risk factors for development of HCC were older age, excessive alcohol consumption, pre-existing T2DM and presence of hepatic iron accumulation. Notably, some studies also suggested that the overall survival in cirrhotic NAFLD patients with HCC was significantly shorter compared with that observed in patients with HCC secondary to HCV cirrhosis (48,52,53). A plausible explanation for this intriguing finding is that cirrhotic NAFLD patients with HCC were older, and more likely to have larger tumor diameters and less surveillance compared to patients with HCC secondary to HCV cirrhosis (48). Conversely, the overall survival of patients with NAFLD-related HCC and neoplastic lesions eligible for curative treatment appeared to be superimposable to that observed in patients with HCC secondary to HCV cirrhosis (48).
Interestingly, however, in the last years the concept that HCC develops only in patients with cirrhotic NAFLD has been challenged, as HCC has been increasingly recognized in non-cirrhotic patients with NASH (11,48,54-57). For example, in a small cross-sectional study of 54 patients with HCC resulting by NAFLD, Leung et al. reported that approximately 15% of them were not cirrhotic, and that non-cirrhotic NAFLD patients had a significantly larger mean tumor diameter at diagnosis compared to those with cirrhosis (58). In another small study of 36 patients without cirrhosis and 47 NAFLD-HCC patients with cirrhosis, Mohamad et al. reported that patients with NAFLD-HCC in the absence of cirrhosis had larger tumor diameters at diagnosis, higher tumor recurrence rates and worse survival outcomes compared to NAFLD-HCC patients with cirrhosis (59). To further increase the complexity of this problem, some cases of HCC in patients with imaging-diagnosed NAFLD (hepatic steatosis) have been also recently described. In a Japanese study involving 6,508 individuals with ultrasound-diagnosed NAFLD and followed for a median of nearly 6 years, Kawamura et al. found that the cumulative rates of HCC were 0.02% after 4 years, 0.20% after 8 years, and 0.51% after 12 years, respectively (60). These authors also observed that patients with advanced NAFLD fibrosis, as detected by aspartate aminotransferase (AST) to platelet ratio index (i.e., a non-invasive clinical score for advanced fibrosis), had an approximately 25-fold increased incidence of HCC compared with those without advanced NAFLD fibrosis (60).
Given that NAFLD is considered to be the “hepatic manifestation” of metabolic syndrome, it is unsurprising that several epidemiological studies also reported a strong association between other metabolic syndrome features, such as overweight or obesity, and the risk of developing HCC. Over the last decade, some large meta-analyses documented that individuals with overweight or obesity had a 50% to 85% increased risk of incident HCC compared with non-obese individuals (61-63). Similarly, in a large follow-up study, including nearly 360,000 individuals from the Prospective Investigation into Cancer and Nutrition Study, Schlesinger et al. reported a strong and positive association between abdominal obesity and HCC incidence over a mean follow-up period of 8.6 years (64).
Putative biological mechanisms linking T2DM and NAFLD with HCC risk
The exact pathophysiological mechanisms linking T2DM, NAFLD and HCC are not completely understood. However, although the pathophysiology underpinning these associations are not entirely clear, understanding of HCC pathophysiology in this context has improved in recent years.
Figure 1 schematically summarizes the putative pathophysiological mechanisms that might link T2DM, NAFLD and HCC.

It is well known that T2DM and NAFLD are strongly associated with increased hepatic/peripheral insulin resistance, lipotoxicity, increased oxidative stress and chronic low-grade inflammatory state. For instance, when insulin resistance and lipotoxicity develop, there is an increased release of multiple pro-inflammatory cytokines (e.g., C-reactive protein, interleukin-1, interleukin-6, tumor necrosis factor-alpha, tumor growth factor-beta), vasoactive factors and pro-oxidant molecules into bloodstream (13,14,19,65). Several studies suggest that all these factors may contribute to the development of HCC by promoting hepatic cellular growth/proliferation and by inhibiting cellular apoptosis (65-71). In addition, in the presence of insulin resistance, insulin concentrations rise in blood, so resulting in increased insulin-like growth factor-1 (IGF-1) production, i.e., a well-known hormone capable to stimulate hepatic cellular growth/proliferation and inhibit cellular apoptosis within the liver. Hyperinsulinemia also stimulates insulin receptor substrate-1 (IRS-1), which plays a key role in the activation of some intracellular cytokine signalling pathways implicated in hepatic carcinogenesis (65,72).
Both T2DM and NAFLD are associated with increased oxidative stress and release of reactive oxygen species (ROS) (64,70,72). Experimental studies reported that when hepatocytes are steatotic, they are able to produce ROS (65,73-76). It is known that increased ROS and oxidative stress may promote the development of many types of cancer, including HCC (65,71,73-76). Indeed, ROS can lead to cytotoxicity, DNA damage as well as activation and suppression of multiple genes that are potentially implicated in the cellular proliferation and growth (e.g., c-Jun amino terminal kinase 1 [c-JNK], p53, D-cycline, c-Fos, c-RAS, and c-Myc), thus further promoting hepatic carcinogenesis (65,71,73-76). In addition, increased production of ROS can be due to mitochondrial dysfunction (i.e., structural mitochondrial lesions, decreased activity of the respiratory chain enzymes and abnormal mitochondrial beta-oxidation) (65,71,73-76). Interestingly, many experimental studies reported that T2DM and NAFLD are associated with the presence of mitochondrial dysfunction, thus resulting in increased ROS production (65,71,73-76). For an updated review on the molecular mechanisms of NAFLD-induced hepatocarcinogenesis the interested readers are referred to Zoller et al. (14).
Recently, accumulating evidence also suggests that gut microbiota alterations might play a part in the pathogenesis of T2DM, obesity and NAFLD (77-80). To note, there is now some evidence suggesting that altered gut microbiota may be also implicated in hepatic carcinogenesis (77-80). The putative pathophysiological processes that link altered gut microbiota and HCC development are complex, but they might include abnormalities in Toll Like Receptors (TLRs), increased levels of gut bacterial metabolites, increased levels of secondary bile acids with subsequent development of intestinal dysbiosis (77-80). With regard to intestinal dysbiosis, for example, in a recent case-control study including 150 HCC patients (105 with early HCC and 45 with advanced HCC) and 131 control individuals, Ren et al. reported that altered gut microbiota (characterized by decreases in Bacteroidetes and increases in Proteobacteria and Fusobacteria) was associated with more advanced HCC (81). It is plausible to assume that alterations in gut microbiota may promote the increased production of multiple fibrotic, inflammatory and cancer growth mediators by the hepatic stellate cells (77-80).
To date, the advent of next-generation sequencing and other “omics” technologies strongly implemented our knowledge(s) about the pathophysiology of HCC (65,71,82-89). For example, several studies reported recurrent mutations in the cell cycle regulator TP53 and CDKN2A, in the gene encoding for albumin, and also in the genes of the β-catenin/WNT signaling pathway (i.e., CTNNB1 and AXIN1) (65,71,82-89). Using the technique of comparative genomic hybridization, other experimental reports documented that patients with NASH have a greater level of genomic instability compared to those without NASH (65,71,82-89). Interestingly, it has been recently reported that some epigenetic alterations might be also important for HCC development (65,71,82-89). For example, the hypermethylation of the E-cadherin-1 (CDH-1) gene has been related to increased incidence of NAFLD-related HCC (65,71,82-89). Accumulating evidence also suggests the importance of genetic variation in the patatin like phospholipase domain-containing protein-3 (PNPLA3) gene (65,71,82-89). This gene encodes for a protein (named adiponutrin) located in intra-hepatic lipid droplets, which is capable to promote hepatic lipogenesis and lipolysis (65,71,82-89). The genetic variations of PNPLA3 gene have been found to be closely associated with increased risk of NAFLD progression, especially with risk of hepatic fibrosis (65,71,82-89). It has subsequently been shown that carriers of PNPLA3 genetic polymorphism encoding for the I148M variant allele are also at higher risk of HCC (65,71,82-89). Therefore, genotyping of the PNPLA3 I148M polymorphism will reveal mechanisms implicated in hepatic fibrogenesis and carcinogenesis and will possibly inform clinical practice in the future (i.e., contributing to identify those to whom HCC surveillance may be targeted).
Epidemiological studies linking the use of certain glucose-lowering medications to HCC risk
Currently, there is increasing evidence to suggest that certain glucose-lowering medications may have a modifying effect on incidence rates of HCC. In 2005, Evans et al. conducted a pilot study suggesting that metformin use may be associated with a lower risk of cancer among individuals with known T2DM (90). After this pioneering study, other larger studies have been performed. For example, in a population-based cohort of 10,309 T2DM patients followed-up for nearly 5 years, Bowker et al. reported that cancer mortality significantly differed among the various treatments for diabetes at baseline: 3.5% for metformin users, 4.9% for sulphonylurea users and 5.8% for insulin users, respectively (91). In a longitudinal study of more than 8,000 patients with T2DM followed for a median of 3 years, Libby et al. showed that those treated with metformin had a lower incidence of total cancer than those treated with sulphonylureas or insulin, independent of age, sex, BMI, hemoglobin A1c, smoking and use of other medications (92). In a study including 610 HCC patients, 618 cirrhotic patients and 1,696 controls, Donadon et al. showed that metformin use was associated with a significant HCC risk reduction compared with the use of sulphonylureas or insulin, in diabetic HCC patients vs. control subjects and vs. cirrhotic patients (93). In a hospital-based study including 420 HCC patients and 1,104 controls, Hassan et al. reported that use of sulfonylureas or insulin conferred the highest HCC risk, whereas use of metformin or glitazones was associated with a 70% risk reduction in HCC in patients with T2DM (94). Similarly, in a large Taiwanese population-based study enrolling 19,349 patients with newly diagnosed T2DM and 77,396 non-diabetic controls, Lai et al. showed that patients with T2DM had a two-fold higher incidence of HCC than those without T2DM, and that this incidence was significantly lower in patients treated with either metformin or glitazones than in those treated with other hypoglycemic agents (95). Some recent meta-analyses confirmed these findings. In a meta-analysis of 5 case-control studies, three cohort studies and two randomized controlled trials with a total of 22,650 HCC cases in 334,307 patients with T2DM, Singh et al. documented that patients treated with metformin had a nearly 50% risk reduction in HCC incidence compared with those treated with sulphonylureas, glitazones or insulin (96). More recently, in a network meta-analysis of 13 randomized controlled trials, cohort studies or case-control studies enrolling approximately 480,000 T2DM patients with more than 240,000 HCC cases, Zhou et al. showed that metformin use was associated with decreased HCC risk, whereas insulin use was associated with a higher risk of this cancer (97).
That said, it is also important to underline that other observational studies produced conflicting results. In a retrospective cohort study of nearly 96,000 individuals with T2DM who began to take metformin or other oral glucose-lowering agents within 12 months of the diagnosis, Tsilidis et al. showed that the users of sulphonylureas or metformin had similar incidence rates of total cancer over 5 years of follow-up (98). Similar results were also found when the analysis was restricted to incidence rates of different types of cancer, such as HCC, colorectal, prostate, lung and breast cancers (98). Interestingly, in a post-hoc analysis of the ADOPT (A Diabetes Outcome Progression Trial) and the RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) trials, Home et al. showed that the cancer incidence rates did not allow to support the evidence that use of metformin conferred a particular protection against cancer risk compared to rosiglitazone (99). In addition, other studies did not support the conclusion that metformin may reduce the rates of cancer incidence among patients with established T2DM (100-102).
Collectively, there is still uncertainty about whether hypoglycemic drugs used for diabetes treatment may either increase or decrease the risk of incident HCC, or even influence cancer prognosis. Most observational studies suggest that metformin use might have any chemo-preventive effects against HCC, and a biologically plausible mechanism also exists (this drug activates AMP-activated protein kinase (AMPK) and inhibits the PI3K/AKT/mTOR signaling pathway that is important in regulating the cell cycle) (1,14,96). In contrast, it is uncertain if the observed insulin-related increase in HCC risk is related to toxicity associated with the medication, or if it is simply reflective of increased HCC risk in patients with more severe diabetes. Further larger randomized clinical trials are required in order to confirm a possible chemo-protective effect of metformin on HCC risk, and to find novel metabolic approaches to HCC prevention and treatment in individuals with T2DM.
Conclusions
HCC incidence is rapidly increasing worldwide, and this is likely linked to the increasing incidence of T2DM, NAFLD and metabolic syndrome. There is now robust evidence of an association between T2DM and HCC development. The pathophysiology underlying development of HCC in this context is complex and is likely to involve NAFLD (especially NASH with varying amounts of hepatic fibrosis), increased hepatic/peripheral insulin resistance hyperinsulinemia, increased pro-inflammatory mediators, oxidative stress, JNK-1 activation, increased IGF-1 activity, altered gut microbiota and immunomodulation. However, a greater understanding of the underlying pathophysiology might help in the future development of rational, targeted treatments for patients with both HCC and T2DM.
In clinical practice, clinicians are increasingly required to manage and treat patients with both T2DM and HCC. Although there are still important gaps in our knowledge(s), the use of metformin may be associated with a lower incidence of HCC. To date, studies reporting on the effect of glucose-lowering medications other than metformin on HCC prognosis are both scant and difficult to interpret, owing to the complexity of pharmacotherapy for T2DM, and the many sources of bias that this complexity may generate. Further research is required to clarify the variables that contribute to the complexity of the associations between T2DM, hyperglycemia, diabetes treatment and HCC risk.
Given that clinicians increasingly encounter patients with coexistent T2DM and HCC, it is desirable that the next randomized clinical trials of new drug candidates in oncology do not exclude people with T2DM in order to better understand the pathophysiological mechanisms linking T2DM and HCC, and to provide new drugs for prevention and treatment of HCC in these patients.
Acknowledgements
G Targher is supported in part by grants from the University School of Medicine of Verona, Verona, Italy.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Klil-Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs:is the fog clearing? Nat Rev Clin Oncol 2017;14:85-99. [Crossref] [PubMed]
- Tuffier T. Diabete et neoplasmes. Archives generales de medecine 1888;7:129-40.
- Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas:global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311-21. [Crossref] [PubMed]
- Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137-149. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma:present and future. Clin Liver Dis 2011;15:223-43. [Crossref] [PubMed]
- Zhang H, Gao C, Fang L, et al. Increased international normalized ratio level in hepatocellular carcinoma patients with diabetes mellitus. World J Gastroenterol 2013;19:2395-403. [Crossref] [PubMed]
- Madkhali AA, Fadel ZT, Aljiffry MM, et al. Surgical treatment for hepatocellular carcinoma. Saudi J Gastroenterol 2015;21:11-7. [Crossref] [PubMed]
- Nair S, Mason A, Eason J, et al. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology 2002;36:150-55. [Crossref] [PubMed]
- Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol 2012;56:704-13. [Crossref] [PubMed]
- Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int 2016;36:317-24. [Crossref] [PubMed]
- Streba LA, Vere CC, Rogoveanu I, et al. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question. World J Gastroenterol 2015;21:4103-10. [Crossref] [PubMed]
- Noureddin M, Rinella ME. Nonalcoholic Fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis 2015;19:361-79. [Crossref] [PubMed]
- Zoller H, Tilg H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism 2016;65:1151-60. [Crossref] [PubMed]
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. [Crossref] [PubMed]
- Lonardo A, Bellentani S, Argo CK, et al. Epidemiological modifiers of non-alcoholic fatty liver disease:focus on high-risk groups. Dig Liver Dis 2015;47:997-1006. [Crossref] [PubMed]
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330-44. [Crossref] [PubMed]
- Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab 2015;100:2231-38. [Crossref] [PubMed]
- Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015;62:S47-S64. [Crossref] [PubMed]
- Sudharsanan N, Ali MK, Mehta NK, et al. Population aging, macroeconomic changes, and global diabetes prevalence, 1990-2008. Popul Health Metr 2015;13:33. [Crossref] [PubMed]
- Leahy S, O'Halloran AM, O'Leary N, et al. Prevalence and correlates of diagnosed and undiagnosed type 2 diabetes mellitus and pre-diabetes in older adults:findings from the Irish Longitudinal Study on Ageing (TILDA). Diabetes Res Clin Pract 2015;110:241-49. [Crossref] [PubMed]
- Kaaks R, Kühn T. Epidemiology:obesity and cancer-the evidence is fattening up. Nat Rev Endocrinol 2014;10:644-45. [Crossref] [PubMed]
- Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012:a population-based study. Lancet Oncol 2015;16:36-46. [Crossref] [PubMed]
- Tsilidis KK, Kasimis JC, Lopez DS, et al. Type 2 diabetes and cancer:umbrella review of meta-analyses of observational studies. BMJ 2015;350:g7607. [Crossref] [PubMed]
- Emerging Risk Factors Collaboration, Seshasai SR, Kaptoge S, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829-41. [Crossref] [PubMed]
- Verlato G, Zoppini G, Bonora E, et al. Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care 2003;26:1047-51. [Crossref] [PubMed]
- Carstensen B, Witte DR, Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia 2012;55:948-58. [Crossref] [PubMed]
- Geier AS, Wellmann J, Wellmann I, et al. Cancer detection rates following enrolment in a disease management program for type 2 diabetes. Diabetologia 2013;56:1944-48. [Crossref] [PubMed]
- Tseng CH. Diabetes and risk of prostate cancer: a study using the National Health Insurance. Diabetes Care 2011;34:616-21. [Crossref] [PubMed]
- Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol 2010;47:87-95. [Crossref] [PubMed]
- Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin 2010;60:207-21. [Crossref] [PubMed]
- Johnson JA, Bowker SL, Richardson K, et al. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia 2011;54:2263-71. [Crossref] [PubMed]
- Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008;371:838-51. [Crossref] [PubMed]
- Lawson DH, Gray JM, McKillop C, et al. Diabetes mellitus and primary hepatocellular carcinoma. Q J Med 1986;61:945-55. [PubMed]
- La Vecchia C, Negri E, D'Avanzo B, et al. Medical history and primary liver cancer. Cancer Res 1990;50:6274-77. [PubMed]
- Fujino Y, Mizoue T, Tokui N, et al. Prospective study of diabetes mellitus and liver cancer in Japan. Diabetes Metab Res Rev 2001;17:374-79. [Crossref] [PubMed]
- El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma:a case-control study among United States Veterans. Am J Gastroenterol 2001;96:2462-67. [Crossref] [PubMed]
- Yu L, Sloane DA, Guo C, et al. Risk factors for primary hepatocellular carcinoma in black and white Americans in 2000. Clin Gastroenterol Hepatol 2006;4:355-60. [Crossref] [PubMed]
- Gao C, Yao SK. Diabetes mellitus:a “true” independent risk factor for hepatocellular carcinoma? Hepatobiliary Pancreat Dis Int 2009;8:465-73. [PubMed]
- El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma:a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006;4:369-80. [Crossref] [PubMed]
- Wang P, Kang D, Cao W, et al. Diabetes mellitus and risk of hepatocellular carcinoma:a systematic review and meta-analysis. Diabetes Metab Res Rev 2012;28:109-22. [Crossref] [PubMed]
- Schlesinger S, Aleksandrova K, Pischon T, et al. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol 2013;24:2449-55. [Crossref] [PubMed]
- Koh WP, Wang R, Jin A, et al. Diabetes mellitus and risk of hepatocellular carcinoma:findings from the Singapore Chinese Health Study. Br J Cancer 2013;108:1182-88. [Crossref] [PubMed]
- Miele L, Bosetti C, Turati F, et al. Diabetes and Insulin Therapy, but Not Metformin, Are Related to Hepatocellular Cancer Risk. Gastroenterol Res Pract 2015;2015:570356. [Crossref] [PubMed]
- Dyal HK, Aguilar M, Bartos G, et al. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients:a systematic review. Dig Dis Sci 2016;61:636-45. [Crossref] [PubMed]
- Sanyal A, Poklepovic A, Moyneur E, et al. Population-based risk factors and resource utilization for HCC:US perspective. Curr Med Res Opin 2010;26:2183-91. [Crossref] [PubMed]
- Ertle J, Dechêne A, Sowa JP, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011;128:2436-43. [Crossref] [PubMed]
- Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci 2016;17:E774. [Crossref] [PubMed]
- Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol 2015;13:594-601.e1. [Crossref] [PubMed]
- White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 2012;10:1342-59. [Crossref] [PubMed]
- Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972-8. [Crossref] [PubMed]
- Guzman G, Brunt EM, Petrovic LM, et al. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med 2008;132:1761-6. [PubMed]
- Reddy SK, Steel JL, Chen HW, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology 2012;55:1809-19. [Crossref] [PubMed]
- Kawada N, Imanaka K, Kawaguchi T, et al. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol 2009;44:1190-94. [Crossref] [PubMed]
- Rahman RN, Ibdah JA. Nonalcoholic fatty liver disease without cirrhosis is an emergent and independent risk factor of hepatocellular carcinoma: A population based study. Hepatology 2012;56:241A.
- Alexander J, Torbenson M, Wu TT, et al. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver:a clinical and pathological study. J Gastroenterol Hepatol 2013;28:848-54. [Crossref] [PubMed]
- Dongiovanni P, Romeo S, Valenti L. Hepatocellular carcinoma in nonalcoholic fatty liver:role of environmental and genetic factors. World J Gastroenterol 2014;20:12945-55. [Crossref] [PubMed]
- Leung C, Yeoh SW, Patrick D, et al. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J Gastroenterol 2015;21:1189-96. [Crossref] [PubMed]
- Mohamad B, Shah V, Onyshchenko M, et al. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int 2016;10:632-9. [Crossref] [PubMed]
- Kawamura Y, Arase Y, Ikeda K, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol 2012;107:253-61. [Crossref] [PubMed]
- Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer 2007;97:1005-8. [PubMed]
- Chen Y, Wang X, Wang J, et al. Excess body weight and the risk of primary liver cancer:an updated meta-analysis of prospective studies. Eur J Cancer 2012;48:2137-45. [Crossref] [PubMed]
- Tanaka K, Tsuji I, Tamakoshi A, et al. Obesity and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol 2012;42:212-21. [Crossref] [PubMed]
- Schlesinger S, Aleksandrova K, Pischon T, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer 2013;132:645-57. [Crossref] [PubMed]
- Wainwright P, Scorletti E, Byrne CD. Type 2 diabetes and hepatocellular carcinoma:risk factors and pathogenesis. Curr Diab Rep 2017;17:20. [Crossref] [PubMed]
- Sakurai T, Maeda S, Chang L, et al. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA 2006;103:10544-51. [Crossref] [PubMed]
- Hoesel B, Schmid JA. The complexity of NF- B signalling in inflammation and cancer. Mol Cancer 2013;12:86. [Crossref] [PubMed]
- Luedde T, Beraza N, Kotsikoris V, et al. Deletion of NEMO/IKK-gamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 2007;11:119-32. [Crossref] [PubMed]
- Li C, Deng M, Hu J, et al. Chronic inflammation contributes to the development of hepatocellular carcinoma by decreasing miR-122 levels. Oncotarget 2016;7:17021-34. [PubMed]
- Giusy E, Poupak F. Hepatocellular carcinoma and CXCR3 chemokines:a narrative review. Clin Ter 2017;168:e37-e41. [PubMed]
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nature Reviews Gastroenterology and Hepatology 2013;10:656-65. [Crossref] [PubMed]
- Ish-Shalom D, Christoffersen CT, Vorwerk P, et al. Mitogenic properties of insulin and insulin analogues mediated by the insulin receptor. Diabetologia 1997;40:S25-S31. [Crossref] [PubMed]
- Rhee SG, Bae YS, Lee SR, et al. Hydrogen peroxide:a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 2000;2000:pe1. [PubMed]
- Yang S, Zhu H, Li Y, et al. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys 2000;378:259-68. [Crossref] [PubMed]
- Spickett CM. The lipid peroxidation product 4-hydroxy-2-nonenal:advances in chemistry and analysis. Redox Biol 2013;1:145-52. [Crossref] [PubMed]
- Hu W, Feng Z, Eveleigh J, et al. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis 2002;23:1781-89. [Crossref] [PubMed]
- Adams LA, Morrison M. The microbiome in obesity, diabetes and NAFLD: what is your gut telling us? Curr Hepatol Rep 2016;15:96-102. [Crossref]
- Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012;21:504-16. [Crossref] [PubMed]
- Darnaud M, Faivre J, Moniaux N. Targeting gut flora to prevent progression of hepatocellular carcinoma. J Hepatol 2013;58:385-7. [Crossref] [PubMed]
- Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013;499:97-101. [Crossref] [PubMed]
- Ren Z, Xu S, Jiang J, Zheng S. A novel diagnosis for early hepatocellular carcinoma based on intestinal microbiome. 21st Annual Meeting of the International Liver Transplant Society, Chicago, IL, 2015.
- Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nature Genet 2015;47:505-11. [Crossref] [PubMed]
- Liu YL, Patman GL, Leathart JB, et al. Carriage of the PNPLA3 rs738409 C>G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol 2014;61:75-81. [Crossref] [PubMed]
- Zain SM, Mohamed R, Cooper DN, et al. Genome-wide analysis of copy number variation identifies candidate gene loci associated with the progression of non-alcoholic fatty liver disease. PLoS One 2014;9:e95604. [Crossref] [PubMed]
- Herath NI, Leggett BA, MacDonald GA. Review of genetic and epigenetic alterations in hepatocarcinogenesis. J Gastroenterol Hepatol 2006;21:15-21. [Crossref] [PubMed]
- Dongiovanni P, Donati B, Fares R, et al. PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol. 2013;19:6969-78. [Crossref] [PubMed]
- Chamoun Z, Vacca F, Parton RG, et al. PNPLA3/adiponutrin functions in lipid droplet formation. Biol Cell 2013;105:219-33. [Crossref] [PubMed]
- Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461-5. [Crossref] [PubMed]
- Falleti E, Fabris C, Cmet S, et al. PNPLA3 rs738409C/G polymorphism in cirrhosis: relationship with the aetiology of liver disease and hepatocellular carcinoma occurrence. Liver Int 2011;31:1137-43. [Crossref] [PubMed]
- Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304-5. [Crossref] [PubMed]
- Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes, who use sulfonylureas or insulin. Diabetes Care 2006;29:254-58. [Crossref] [PubMed]
- Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer:a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620-5. [Crossref] [PubMed]
- Donadon V, Balbi M, Mas MD, et al. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int 2010;30:750-8. [Crossref] [PubMed]
- Hassan MM, Curley SA, Li D, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer 2010;116:1938-46. [Crossref] [PubMed]
- Lai SW, Chen PC, Liao KF, et al. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol 2012;107:46-52. [Crossref] [PubMed]
- Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and the risk of hepatocellular cancer:a systematic review and meta-analysis. Am J Gastroenterol 2013;108:881-91. [Crossref] [PubMed]
- Zhou YY, Zhu GQ, Liu T, et al. Systematic review with network meta-analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci Rep 2016;6:33743. [Crossref] [PubMed]
- Tsilidis KK, Capothanassi D, Allen NE, et al. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care 2014;37:2522-32. [Crossref] [PubMed]
- Home PD, Kahn SE, Jones NP, et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010;53:1838-45. [Crossref] [PubMed]
- Stevens RJ, Ali R, Bankhead CR, et al. Cancer outcomes and all-cause mortality in adults allocated to metformin:systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia 2012;55:2593-603. [Crossref] [PubMed]
- Thakkar B, Aronis KN, Vamvini MT, et al. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients:a meta-analysis using primary data of published studies. Metabolism 2013;62:922-34. [Crossref] [PubMed]
- Yin M, Zhou J, Gorak EJ, et al. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist 2013;18:1248-55. [Crossref] [PubMed]


