Ablation of long-standing persistent atrial fibrillation
Introduction
Atrial fibrillation (AF), the most commonly encountered arrhythmia, is defined as a disorganization in the electrical impulses of the upper chambers of the heart resulting in an irregular heartbeat leading to an interrupted blood flow from atria to the ventricles (1). Currently AF affects a population of 2.7 to 6.1 million adults in United States. Almost 2% and 9% of adults below and above the age of 65, respectively have AF (2). The annual cost of AF is estimated to be $6 billion (3,4). The economic impact of this debilitating condition continues to increase with the aging population and the ever increasing comorbidities such as hypertension, diabetes mellitus, heart failure, chronic kidney disease, ischemic heart disease, obesity and hyperthyroidism (4).
Classification
For standardization, AF is further categorized into paroxysmal, persistent or longstanding persistent according to the guidelines stated by American College of Cardiology, European Society of Cardiology and American Heart Association (5). Paroxysmal AF occurs when a patient has at least two recurrent episodes that self-terminate within 7 days. An AF episode is defined as one that is documented by electrocardiogram (ECG) monitoring and has a duration of at least 30 seconds, or if less than 30 seconds, is present continuously throughout the ECG monitoring tracing. Paroxysmal AF also includes the patient population in which cardioversion is performed within 48 hours of onset. Persistent AF occurs when the arrhythmic episodes endure beyond 7 days or require cessation with pharmacological or direct current cardioversion between 48 hours to 7 days duration. Continuous incidences of AF extending greater than 12 months are classified as longstanding persistent (5-7). Silent AF is characterized as an incidental finding on ECG or rhythm strip in an asymptomatic patient. Once identified, it can then be additionally characterized into any of the above listed categories (5). Furthermore, patients being considered as a candidate for catheter/surgical ablation for AF should be reclassified and not listed as permanent AF. This term remains exclusive for patients in which it is determined not to restore sinus rhythm by any technique (5).
Goal of review
Successful ablation of long-standing persistent atrial fibrillation (LSPAF) has been a challenging task for many electrophysiologists. As per current joint American Heart Association, American College of Cardiology and Heart Rhythm Society guidelines, catheter ablation may be considered for symptomatic LSPAF prior to initiation, or if refractory/intolerant to, at least one class I or class III anti-arrhythmic medication (class IIb indication) (3). The goal of this review is to discuss the mechanisms that maintain LSPAF, the various catheter ablation strategies that are currently being utilized, and their success with short- and long-term freedom from recurrent atrial arrhythmias. Many of the studies that will be discussed assess various substrate modification and trigger ablation strategies in patients with various stages of AF however most studies combine the analyses of success in patients with persistent AF and LSPAF. Given this limitation, we will focus on LSPAF.
Mechanism
Early in the clinical process, the pulmonary veins (PV) (5,8) remain the main site of ectopic foci that initiate AF which explains a success rate as high as 85% with pulmonary vein isolation (PVI) catheter ablation (6) (Figure 1). As AF evolves from paroxysmal to persistent, as a result of complex underlying electro-structural modifications, the success rate begins to decrease (5). In 1959, Moe et al. presented one of the prevailing theories explaining the concept of AF: the multiple wavelet hypothesis (9). AF propagates through existing wavelets which in turn are dependent on diminished conduction, reduced refractory periods and an enlarged atrial mass. The localized source hypothesis cultivates the idea of rotors or focal impulses as the leading etiology of disorganized AF (10,11). In 1994, Haïssaguerre and colleagues laid down the foundation of focal mechanisms of AF mainly located in the PV (12). Later, Ho et al. (13) also identified the cardiac tissue sleeves in the PV as a possible origin of ectopic foci by shedding some light on the long forgotten anatomical structures initially demonstrated by Nathan and Eliakim in 1966 (14). The main pathophysiological mechanisms giving rise to AF are electrical and structural alterations in atrial tissue as well as effects on of the autonomic nervous system (7).
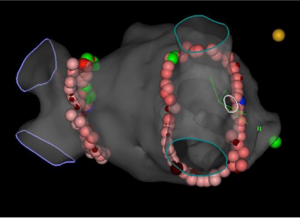
Electrical remodeling
Various medical conditions such as atrial tachyarrhythmias and heart failure make the atria susceptible to AF. Normal resting potential is maintained by the large number of potassium channels (IK1) in atrial cells. The key mechanisms causing focal atrial arrhythmias are enhanced automaticity, early afterdepolarization (EAD) and delayed afterdepolarizations (DAD) (7). Automaticity, or spontaneous depolarization, does not occur in normal atrial myocardial cells (15). Enhanced automaticity occurs when there is an imbalance of potassium channels (IK1) and pacemaker current (If) or when there is decreased capacity of IK1 channel to transport potassium ions (7,15). Excessive prolongation of action potential duration causes the development of EAD. It involves early activation of calcium channel [L-type Ca (I CaL)] during the repolarization phase allowing inward entry of calcium ions (7,16). Conditions predisposing to EAD include injured myocardial tissue, heart failure, electrolytic disturbances, hypoxia, acidosis and antiarrhythmic agents (15). DADs occur when there is an abnormal diastolic outflow of calcium from sarcoplasmic reticulum calcium stores as a response to transmembrane calcium entry. An excess of diastolic calcium ensues an exchange of a single calcium ion for three extracellular sodium ions creating a net positive environment by transient inward current (Iti) that creates DADs (7,16). Repetitive DADs lead to focal atrial tachycardias (16). DADs are usually seen during digitalis toxicity and elevated catecholaminergic levels (15). Hence electric remodeling due to underlying atrial conditions can contribute to progression of AF from a paroxysmal to a persistent form.
Autonomic remodeling
Autonomic remodeling or interplay between the sympathetic and parasympathetic systems can also generate ectopic foci within the left atrium (LA) to create and propagate AF (17). Both animal and human models alike have shown an association between AF and abnormal autonomic system function. Numerous autonomic ganglia are located in the LA with the highest concentration within 5 mm of PV-LA junction. Approximately 30% of ganglionic cells have both adrenergic and cholinergic properties (18). Vagal discharge by parasympathetic system and sympathetic activation leading to a heterogeneous increase in transmembranous and intracellular calcium and ultimately the release of norepinephrine, which can trigger focal areas in the LA and differentially decrease the atrial effective refractory period across the posterior LA, contribute to the initiation and preservation of AF (17).
Ablation of the ganglionated plexi overlying the LA has shown some promise in patients with paroxysmal AF. Pappone et al. performed circumferential PV ablation in 297 patients with paroxysmal AF; 34.3% of patients underwent additional vagal denervation (19). At 1 year follow up, patients who received additional vagal denervation were found to have less recurrence of AF compared to their counterparts. Katritsis et al. randomized 242 patients in his prospective study to PVI alone, ganglion plexus (GP) ablation alone or combined PVI-GP ablation (20). At 2 years follow up, freedom from atrial arrhythmia was 74%, 56%, 48% (P=0.004) in the PVI-GP, PVI-alone, and GP-alone cohort, respectively. Conversely, outcomes with PVI-GP ablation seem to not be as favorable in patients with persistent or LSPAF. In 2013, Zheng et al., revealed at approximately 5 years follow up, the success rate for surgical PVI-GP ablation was 51.8% for paroxysmal AF, 28.2% for persistent AF, and 28.6% for LSPAF (21). Thus, the influence of the autonomic nervous system on LSPAF remains uncertain.
Structure remodeling
There is growing consensus that fibrosis in the atria ranging from mild to severe can lead to significant advancement in the disease process of AF. Enhanced expression of collagen I in the LA can lead to excessive accumulation of collagenous material in the extracellular space, especially in those with lone AF (22). Platonov et al. studied the post-mortem atrial tissue samples obtained from posterior left atrial wall, at the level of the superior PV, inferior PV, Bachmann’s bundle and crista terminalis (23). Interestingly, three to five times greater fibrosis and fatty infiltration was noted compared to patients with no history of AF, regardless of tissue sampling site. Furthermore, the extent of fibrosis was higher in the permanent versus paroxysmal AF cohort. These findings were similar to Kuppahally et al. who demonstrated significantly more fibrosis in patients with persistent AF compared to patients with paroxysmal AF (22%±17% vs. 14%±9%, P=0.04) (24).
Stiles et al. in his small study of 25 patients successfully demonstrated the role of structural remodeling in AF (25). Patients with paroxysmal AF had reduced conduction velocity, longer effective refractory periods and much lower voltages as a result of abnormal atrial substrate which promoted progression of AF. Similar conclusions were also drawn by Teh et al. when they described an electroanatomic substrate in the LA beyond the focus of the PV in AF patients without any evidence of structural heart disease (26). In fact, patients with persistent AF were more likely to have a greater proportion of low voltage, slower conduction and increased area of complex signals compared to paroxysmal AF group. Structural atrial remodeling has also been observed at a cellular level in animal models as a result of rennin-angiotensin system activation (6). A study in transgenic rats demonstrated that stretching of the atrial musculature leads to release of angiotensin II and transforming growth factor-B1which in turn stimulates interstitial fibrosis and dilatation of atria (27). It remains unclear if atrial fibrosis is a cause or consequence of AF.
Evidence from numerous studies now helps us understand some of the fundamental points of structural remodeling. First, fibrosis helps AF persist and thus facilitates the transformation from paroxysmal to persistent. Second, as AF transitions from paroxysmal to persistent, the location of the substrate for AF gradually shifts away from the PV which may ultimately guide the ablation approach for each individual patient.
Typically, patients with hypertension are thought to be more vulnerable to development of AF given its association with atrial fibrosis. Uncontrolled systemic hypertension can lead to LA remodeling due to increased pressure and volume overload. Not surprisingly, the relationship between average systolic blood pressure and AF is stronger for persistent AF compared to paroxysmal AF (28). The atrial remodeling that is associated with systemic hypertension has been also characterized by global conduction slowing, regional conductional delays and increased AF inducibility (29). Mahnkopf et al. compared LA structural changes in lone AF patients to the patients with common conditions like hypertension, diabetes mellitus, congestive heart failure, stroke and coronary artery disease through delayed enhancement magnetic resonant imaging (DE-MRI) (30). They found that the extent of LA remodeling was totally independent of the most common comorbidities and the type of AF. Oakes et al. had a similar observation in their study of 81 patients demonstrating a high degree of variability in LA structural remodeling regardless of the category of AF (31).
Complex fractionated electrograms
Complex fractionated electrograms (CFAE) was first described in 2004 by Nademanee et al. (32). While there is much debate as to what qualifies as CFAE, many investigators define them as continuous atrial activity, complex fractionated potentials or low voltage electrograms with a short cycle length of less than 120 milliseconds over a 10-second period (32-34) (Figure 2). Gerstenfeld studied the mechanism of CFAE in canine models. He described wavefront collision as the most common form of activation in the region of CFAE (48%) whereas abnormal conduction through areas of functional block and reentry phenomenon were responsible for the remainder of CFAE. The majority of the multiple wavelets were found to be circulating between posterior LA and entry/exit region of PV (35). Konings et al. demonstrated that CFAE originate along the regions of slow conduction, functional block, and pivot points (33). All three factors facilitate the creation and persistence of AF. Areas of slow conduction reduce the wavelength of wavelets and increase their number in atria while the lines of conduction block disintegrate the wavelets and the pivot points ensure timely rotation of the wavelets to prevent their termination at the atrial boundaries. These abnormal conduction patterns in the atria, if identified, can serve as a potential target for ablation.

Role of rotors
Rotors are defined as regions of functional reentry responsible for driving AF (36). Davidenko et al. first demonstrated the self-sustaining rotating waves, introduced by delivering a single premature electrical stimulus in a normal epicardial myocardium (11). Once instigated, this gives rise to an infinite circulation of self-propagating waves which can be dissipated only by providing a precisely timed electrical stimulus. Decades of similar studies have proven that rotors are a primary phenomenon rather than a secondary event disseminating spiral waves into the surrounding the tissue (37). Rotors are enabled by the ionic and conduction defects at the cellular level and intermittent areas of fibrosis lead to stabilization of rotors (36,37). Techniques such as optical mapping, epicardial contact electrodes with high spatiotemporal resolution assist in revealing the location of rotors hence making them potentially amenable for ablation (38,39). However, the importance of rotors to human AF remains controversial. More recently, high resolution mapping has shown some evidence that dissociated fibrillation waves originating from different directions on the endo-epicardial surface may act as the main substrate of LSPAF (40). Nevertheless, additional investigations into underlying mechanism of propagation of LSPAF are crucial to the development of successful ablation strategies.
Ablation strategies
PVI has been shown to be a very effective ablation technique in patients with paroxysmal AF but may not be as effective by itself in patients with persistent or LSPAF (41,42). The reason for contrasting success rates is likely due to the different mechanisms, additional triggers and substrate that maintain persistent AF. We will now discuss the adjunctive catheter ablation techniques including linear lesions, modulation of rotors and focal impulses, CFAEs, substrate modification including assessing atrial fibrosis, isolation of left atrial appendage, and surgical based approach.
Linear lesions
Linear ablation has been utilized to create electrical barriers that prevent single macro-reentrant wavefronts from circulating. Swartz et al. initially performed RF ablation with multiple long linear ablation lines in both atria deemed as an endocardial replica of the MAZE procedure (43,44). In 1999, Ernst et al. investigated the safety and efficacy of biatrial linear ablation versus right atrium (RA) ablation only which included an isthmus line between the inferior vena cava (IVC) and tricuspid annulus, an anterior line between the superior vena cava (SVC) and tricuspid annulus, and an intercaval line between the ostia of the IVC and SVC in patients with idiopathic AF (45). Due to difficulty in creating a left atrial lesion set connecting the PV ostia with mitral annulus (MA), 100% recurrence was noted in the biatrial arm and 94% recurrence in the RA ablation arm despite a complete linear lesion set in 56% of patients, concluding that successful linear ablation in the RA does not prevent AF recurrence. In 2003, Ernst et al. investigated the efficacy of linear lesions within the LA in patients with intermittent or chronic symptomatic AF (mean duration 10±8 years), assessing various strategies including (I) circular lesion around all PV ostia and a connection to MA; (II) three linear lesions, including a roof line from right superior to left superior (LS) PV, ablation from mid-roof line to anterior MA, and from roof line to posterior MA; (III) separate encircling lesions around the right and left PV with linear lesions along the posterior LA wall and towards the lateral MA; and (IV) encircling lesions as in set C but without the linear lesions (46). Despite the inability to achieve conduction block across linear lesions in many patients, 74% of patients were found to have freedom from AF at long-term follow up (620±376 days). Not only did this study reinforce the difficulties in creating long linear lesions and its propensity for LA macroreentry due to conduction gaps, but that durable PVI at a minimum is vital in successful termination of AF.
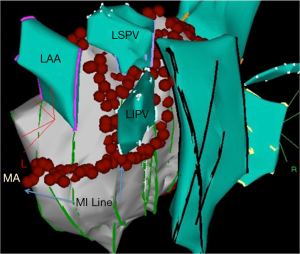
Jaïs et al. evaluated PVI and cavotricuspid isthmus (CTI) linear ablation in 100 patients with drug refractory symptomatic AF and examined its efficacy compared to 100 patients undergoing the same procedure plus linear mitral isthmus (MI) ablation [lateral MA to left inferior (LI) PV] (47) (Figure 3). At 1 year follow up, this prospective non-randomized study revealed 87 patients with MI ablation in sinus rhythm compared to 69 patients without (RR for AF recurrence 0.2; 95% CI 0.1–0.4; P<0.001). In their prospective randomized study including 187 patients, Fassini et al. also were able to demonstrate that the addition of MI line compared to PVI alone was more successful in maintaining sinus rhythm at 1 year follow up (71%±5% vs. 53%±5%, P<0.01) especially in patients with persistent AF (48). Feasibility of roofline ablation, linear block between the right and LS PVs, was initially studied by Hocini and colleagues in 2005 (49). Ninety patients with drug refractory paroxysmal AF were divided into two cohorts: empiric LA roof linear ablation plus PVI and PVI alone. At 15±4 months follow up, significantly more patients in the roofline group were arrhythmia-free compared to those with PVI alone (87% vs. 69%, P=0.04). Roofline ablation also resulted in an increase in the fibrillatory cycle length, termination, and subsequent non inducibility of AF. Wu et al. investigated whether a pure extensive linear ablation including roofline, MA and CTI without PVI was associated with better long term outcome in 120 consecutive patients with drug refractory persistent AF (50). Despite initial successful ablation and conversion to sinus rhythm in 62.5%, a median follow up period of 5.1 years demonstrated that sinus rhythm was maintained without antiarrhythmic drugs in only 40% of patients.
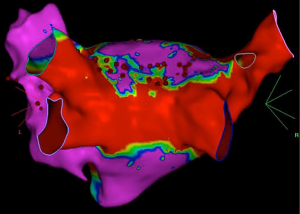
CFAE
In 2004, Nademanee et al. suggested ablation of AF through a CFAE-based approach (32). Out of 121 patients enrolled, 76% achieved freedom from AF at 1 year follow up after one procedure, and 91% after up to two procedures. Although standard PVI was not performed, most of the CFAE targeted for ablation originated in PV. While these initial results were promising, many other investigators were unable to achieve the same success.
Oral et al. performed CFAE ablation in the LA and coronary sinus in 100 patients with persistent AF; freedom from AF was only 33% at 14±7 months follow up (51). In 2009, Oral et al. then randomized 100 patients with LSPAF who had failed antral PVI to either have additional CFAE ablation or cardioversion (50 in each group) (52). At 10±3 months follow up, 36% vs. 34% (P=0.84) were in sinus rhythm in the cardioversion and CFAE groups, respectively. The Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial (STAR-AF), a randomized control multicenter international study in 2010, performed head-to-head comparison of CFAE vs. PVI vs. PVI plus CFAE in 100 patients with either high burden paroxysmal AF or persistent AF (53). At 1 year follow up, 74% of patients in CFAE plus PVI group remained in sinus rhythm after their first procedure which increased to 88% after two procedures. In contrast, successful ablation was reported in only 29% in the CFAE-only cohort which improved to 38% after two procedures. A meta-analysis comprising of 8 studies and 760 patients revealed a statistically significant freedom from AF in CFAE and PVI cohort in persistent AF patients compared to paroxysmal AF (54). However, in 2015, Providência et al. performed a meta-analysis of 13 studies involving 1,415 patients and revealed PVI plus CFAE revealed no superiority over the standard PVI procedure in patients with paroxysmal or persistent AF patients (55). Estner et al. also compared CFAE technique to linear ablation in addition to standard PVI in 116 patients with persistent AF (56). Both CFAE and linear ablation cohorts yielded similar results in terms of freedom from atrial tachyarrhythmias at 1 year follow up (56% vs. 54%, P=0.8). Nonetheless a greater recurrence of AF was detected in PVI plus linear ablation group compared to patients assigned to PVI and CFAE ablation in whom atrial tachycardia was more commonly seen.
CFAE ablation beyond PVI does not necessarily enhance single procedure efficacy in ablating persistent or LSPAF. The RASTA study demonstrated that the addition of LA CFAE ablation with PVI was less efficacious to both PVI plus ablation of non-PV triggers using a stimulation protocol (standard approach) as well the standard approach plus empirical ablation of common non-PV AF trigger sites (MA, fossa ovalis, eustachian ridge, crista terminalis, and SVC) (57). Interestingly, patients who underwent repeat ablation for arrhythmia recurrence had at least 1 PV reconnection (≥3 PVs in most subjects). Targeting these sites alone resulted in excellent long term (80%) AF control rates suggesting that durable PVI should be the cornerstone when addressing ablation in persistent or LSPAF. The STAR-AF II trial compared the approach of PVI plus CFAE ablation vs. other standard ablation strategies [PVI plus linear lesions (LA + MI) vs. PVI alone] in a randomized fashion with patients in persistent AF (41). The trial concluded that there was no statistically significant reduction in the rate of recurrent AF with the addition of linear or CFAE ablation to PVI alone after the initial blanking period and at 18 months follow up. The addition of CFAE and linear ablation resulted in longer procedure time, increased fluoroscopy exposure and slightly worse procedural adverse effects. Recently, Kim et al. investigated the combination strategy of combining PVI and linear lesions followed by CFAE ablation in patients with LSPAF (58). Interestingly, his prospective randomized study revealed that additional CFAE ablation resulted in a numerically (but statistically insignificant) higher AF recurrence compared to PVI + linear lesions (32.1% vs. 18.5%, P=0.166).
The basis for conflicting data on the CFAE lies in the fact that CFAE can be a nonspecific indicator of key target sites (34). All CFAEs are not equivalent, and they can be created by passive mechanisms that do not play an active part in propagation of AF (34,59). Further investigation into distinguishing passive versus active CFAE sites may lead to more promising outcomes.
Focal impulse and rotor modulation (FIRM)
As mentioned above, rotors and focal impulses have been suggested by some investigators to be localized sources that sustain paroxysmal, persistent and LSPAF. FIRM had initially shown promise to improve the outcome of AF ablation in paroxysmal and persistent AF patients with the intention of eradication of local sources and rotors on repeat FIRM mapping (60). The Conventional Ablation for Atrial Fibrillation with or without Focal Impulse and Rotor Modulation (CONFIRM) trial, a non-randomized prospective study, revealed that bi-atrial FIRM guided ablation prior to conventional (PVI) ablation resulted in an 85% success rate with acute termination of a mixed population AF vs. 20% with conventional ablation alone (61). Narayan et al. later published the long term efficacy of FIRM guided ablation in the 3 years follow up of the CONFIRM trial: freedom from AF after 890 days was 77.8% vs. 38.5% in those who received FIRM-guided plus conventional ablation and conventional PVI alone, respectively (62).
In 2014, Miller et al. performed a prospective registry study of mixed AF patients (9% LSPAF) who underwent FIRM-guided ablation followed by conventional PVI ablation at 10 experienced ablation centers (63). AF sources were detected in 100% of patients with each having an average of 2.3±0.9 rotors or focal sources. Interestingly, patients with persistent AF had a higher number of atrial AF sources compared to those with paroxysmal AF, reinforcing the idea that rotor and focal impulses play a large role in sustaining non-paroxysmal AF. At 1 year follow up, freedom from AF as a result of single procedure was observed in 80.5% (62/77) of patients and 87.5% of patients who had no prior ablation procedure (35/40; n=32 persistent/longstanding persistent). However, these outcomes were not statistically significant (18/23, 78.3% vs. 44/54, 81.5%, P=0.89) between patients who suffered from paroxysmal versus persistent AF, respectively. Spitzer et al. published successful long term outcomes with FIRM guided combined with conventional ablation (PVI) in patients with non-paroxysmal AF (83% LSPAF) who had failed prior conventional ablation (64). At 6 and 12 months follow-up, 73.2% and 76.9% of patients were free from AF/AT, respectively. Miller et al. recently reported favorable long term outcomes noted in their registry of patients who had undergone FIRM-guided ablation (65). This non-randomized single center study involved 170 patients with paroxysmal and non-paroxysmal AF (32% LSPAF) who underwent FIRM guided ablation followed by PVI (95% of patients). With regards to patients with LSPAF, the combination of FIRM-guided and conventional ablation resulted in acute termination of AF in 19% of patients. At 1 year follow up, 82% of patients with LSPAF had freedom from AF without AAD after a single procedure and 57% with freedom from all atrial arrhythmias.
Despite the promising results of the above mentioned non-randomized studies of FIRM ablation, they have not been validated by independent investigators or with randomized clinical trials. In 2016, the observational study published by Buch et al. revealed poor long-term outcomes with FIRM-guided ablation in patients with paroxysmal and persistent AF; only 37% of patients were free from AF at 18±7 months follow up (66). Steinberg et al. also demonstrated low long-term efficacy with FIRM-guided ablation in various AF classes in their single-center observational study (67). FIRM ablation was performed in 43 patients (72% with median of one prior PVI), followed by redo PVI in 31/43 patients. At 16±10.7 months follow up, nine (21%) were free from AF. One could argue the success of the FIRM-guided ablation studies with favorable outcomes are attributed to adjunctive PVI. As mentioned multiple times in this review, PVI remains the foundation to all AF catheter ablations. Given these studies are non-randomized with no PVI control group, it is difficult to conclude that FIRM guided ablation is the answer. Furthermore, the signal processing algorithms used for FIRM mapping by the only system commercially available (Topera Solution, Abbott, Menlo Park, CA) has not been fully revealed its manufacturer. Additionally, the 64-pole basket catheter generally covers about 50% of the atrial surface area, calling into question the ability of the system to identify rotors on the order of a few cm2. As a stand-alone ablation methodology for non-paroxysmal AF, FIRM guided ablation alone has not been shown to be efficacious (68).
Substrate ablation
Recently considerable work has been done to explore atrial scar sites as a possible arrhythmic substrate responsible for preservation of AF. As mentioned earlier, AF induces electrophysiologic and cellular changes which result in its persistence (“AF begets AF”) (8,69). Delayed enhancement magnetic resonance imaging (deMRI) has been used to assess LA fibrosis prior to ablation, and quantify ablation induced scarring. A single center study revealed that the degree of circumferential PV scar has been inversely correlated to procedure success (70). The feasibility of quantifying atrial fibrosis prior to AF using deMRI was investigated in the deMRI-guided Fibrosis Ablation vs. Conventional Catheter Ablation of Atrial fibrillation (DECAAF), a multicenter, prospective observational cohort study. Arrhythmia recurrence was associated with the degree of left atrial wall fibrosis: for every 1% increase in fibrosis, HR was 1.06 (95% CI 1.03–1.08; P<0.001) (71).
Catheter ablation based on individual scar distribution is the emerging approach for treatment of persistent AF. In 2005, Verma and colleagues identified patients with areas of scar in LA and showed that these patients had higher incidence of AF recurrence (57%) when compared to patients with no left atrial scar (19%). Additionally, these patients had larger LA size, lower ejection fraction, greater levels of inflammatory markers, and lower success rates with catheter ablation.
Rolf et al. correlated low voltage areas in LA (<0.5 mV) to LA scarring and found a higher scar burden amongst patients with persistent AF (35%) compared to the patients with paroxysmal AF (10%) (72). They reported a 70% success rate of ablation with targeting of LA substrate with low voltage areas in addition to PVI, in comparison to only 26% in patients where PVI was the sole target.
In a recent single center retrospective study, voltage-guided ablation therapy was employed to the posterior wall of LA in addition to PVI versus PVI alone; 80% of the patients in PVI plus LA posterior wall group were free from AF in contrast to 57% of the patients with PVI alone (73). Yamaguchi et al. revealed that low voltage based substrate modification in addition to PVI is more efficacious in patients with persistent AF with low voltage zones (74). Yang and colleagues targeted PV and CTI for ablation in 86 patients with nonparoxysmal AF; 70% of the patient population were recognized to have low voltage areas and hence received additional ablation (75). After 30 months follow up period, 69.8% of the patients were able to maintain sinus rhythm compared to 51.3% in whom no further procedures were performed. Studies suggest higher LA scar burden enable persistence of AF and targeting of these areas may hold promise of freedom from AF (76).
Another novel ablation strategy called box isolation of fibrotic areas (BIFA) utilizes electroanatomic voltage mapping (EAVM) to identify fibrotic LA substrate. This new concept was initially tested successfully by Kottkamp et al. in patients with paroxysmal AF who required redo procedures despite durable PVI. After performing standard PVI and spontaneous or electrical cardioversion, low voltage areas were mapped, isolated and ablation lines were connected to initial PVI lines. Next, he applied BIFA plus PVI to patients with non-paroxysmal AF who had low voltage areas detected by EAVM and achieved a successful rate of 72.2% single procedure freedom from AF/AT (77,78). Patients with no detectable low voltage areas achieved comparable success with PVI alone. This personalized approach to ablation stresses the importance of durable PVI as well as EAVM in paroxysmal and non-paroxysmal AF.
Empirical electrical isolation-left atrial appendage (EEI-LAA)
Non-PV targets such as SVC, ligament of Marshall, coronary sinus, crista terminalis, and left atrial posterior wall as a sources for initiating and maintain AF have been of interest. Another non-PV target and the most common source of thrombus formation in patients with AF-related thromboembolism is the LAA (3). In 2010, Di Biase et al. revealed that LAA firing was observed in 266/987 (27%) patients with AF referred for redo ablation, with LSPAF being the most prevalent (58%) (79). EEI-LAA compared to standard PVI ablation resulted in a 15% versus 74% (P<0.001) recurrence of AF/AT at 12±3 months follow-up, respectively. Further stratifying those who had recurrence and EEI-LAA, LSPAF was noted to have 68% recurrence, compared to 28% in persistent AF and 4% in paroxysmal AF which reinforced the difficulty in ablating LSPAF. The recent randomized study, BELIEF, investigated the effectiveness of additional empirical electrical LAA isolation compared to standard PV plus non-PV trigger ablation in LSPAF (80). At 12 months follow up, 56% of patients who had received EEI-LAA had freedom of atrial arrhythmias compared to 28% with standard PV + non-PV ablation. Even at 24 months follow up with an average of 1.3 procedures, freedom from arrhythmia was noted to be 76% in those who received EEI-LAA vs. 56% with standard PV + non-PV ablation. Repeat ablation was performed in both cohorts: 27/37 (73%) in EEI-LAA group and 35/63 (56%) in the standard ablation group. Of note, PV reconnection was noted to be similar in both groups (11.1% vs. 11.4%, P=1.0). Despite additional mean radiofrequency ablation (RFA) time of 16 min, EEI-LAA did not result in a high procedural complication rate. Isolation of LAA can be performed electrically or potentially with a mechanical closure system. In 2015, Lakkireddy et al. studied the impact of LAA isolation with the percutaneous endo-epicardial LARIAT (SentreHeart, Redwood City, CA) procedure prior to standard PVI ablation in patients with persistent AF (mean AF duration of 52 months) (81). Freedom from atrial arrhythmias at 12 months post-ablation was higher in those who received the additional LARIAT procedure compared to the standard ablation group (65% vs. 39%; P=0.002). The prevalence of LAA as a trigger for AF is still quite low and ablation of this specific source may not apply to all. A potential issue of LAA-EIA is that the LAA will no longer contract if isolation is durable, resulting in stasis and lifelong anticoagulation or requirement for closure. Another limitation to this approach is the risk of perforation if ablation is performed within the LAA.
Hybrid approach
Surgical modalities have been utilized to treat patients with symptomatic drug refractory long standing AF. As per current guidelines, stand-alone surgical ablation is a Class IIb recommendation in highly symptomatic AF patients intolerant of other approaches (3). James Cox’s original “cut-and-sew” surgical maze procedure (82) which was developed to treat this specific population now has evolved into the Cox-maze IV surgery (83). The basis of the maze procedure is to create lines using RFA, cryoablation, and surgical incisions in order to partition atrial tissue to prevent the maintenance of re-entrant wavelets. Soon thereafter the epicardial-only technique was born, using only RFA alone, avoiding cryoablation and surgical incisions. This minimally invasive approach would not require cardiopulmonary bypass surgery and be able to address the LA using mini-thoracotomies, thoracoscopy and/or robotics. Similar to catheter ablation, the success of the epicardial-only approach is greater with paroxysmal versus persistent AF. Edgerton et al. demonstrated using a minimally invasive surgical approach to perform bilateral PV antral isolation and targeted partial autonomic denervation resulted in 83.7% vs. 56% freedom from AF at 6 months in patients with paroxysmal AF and persistent/long-standing persistent AF, respectively (84).
In order to improve its efficacy, the hybrid surgical and catheter ablation approach was developed. The benefits of combining endocardial catheter and epicardial surgical ablation include the formation of transmural lesions which would be beneficial in the setting of excessive scar or increased structural remodeling, and addressing the conduction gaps in lesions caused by epicardial ablation. Zembala et al. investigated the safety, feasibility, effectiveness of the hybrid approach in 70 patients at with persistent and LSPAF (85). Freedom from arrhythmia at 6 and 12 months post-procedure was 78.3% and 84.1%, respectively. Geršak et al. reported similar success with convergent procedure for treatment in 76 patients with paroxysmal and non-paroxysmal AF and at 4 years follow up, 81% of patients were in sinus rhythm (86,87). Potential benefits of convergent procedure include less radiation exposure, shorter electrophysiology catheter-based procedure, reduction in the likelihood of esophageal injury that may be caused by endocardial ablation. However, the procedure is more invasive than purely endocardial catheter-based ablation strategies (85). Catheter ablation can be performed immediately after the surgical thoracoscopic procedure, or months later (Figure 4). A benefit of performing both procedures during same setting is no requirement for a second administration of anesthesia or separate hospitalization. However, allowing enough time after surgical ablation would allow reconnections or conduction gap to become more apparent and thus potentially more successfully ablated. Bisleri et al. utilized the sequential staged hybrid approach in 45 patients with LSPAF with catheter ablation occurring at least 1 month post-surgical ablation. Freedom from AF was noted in 88.9% (40/45) of patients at 28 months follow up (88).
Conclusions
Ablation of LSPAF is a challenging task; the success of single procedure or different combination of therapies is variable and not necessarily reproducible. Is better? Combining multiple strategies and/or the necessity for multiple ablation procedures result in potentially longer fluoroscopy times, increased risk of complications, and increased risk for creation of substrate for future atrial arrhythmias. Review of multiple trials and observation studies have led to the conclusion that durable PVI is important irrespective of AF classification. The reason why some patients required redo ablation procedures is often due to PV reconnection and development of non-PV triggers. A tailor-made approach utilized for each patient based on type of AF and presence/location of substrate may offer the best approach for LSPAF. The role of deMRI to assess for atrial fibrosis prior to performing catheter ablation continues to evolve and may allow for more effective prognostication and pre-procedure planning. Empiric lesion sets appear to be ineffective and possibly proarrhythmic. As substrate identification becomes more accurately defined, substrate modification will likely supplant these empiric approaches. Additionally, the collaboration between electrophysiologists and surgeons may offer further evolution of techniques and technologies to help tear the very difficult LSPAF population. The safety and efficacy of the above techniques still needs to be evaluated with prospective randomized trials in order to gain a better understanding of the various targets for LSPAF.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Jacobson is consultant for CARTO and St. Jude Medical; Dr. Iwai is part of the Speakers Bureau for Biosense Webster (CARTO); the other authors have no conflicts of interest to declare.
References
- Centers for Disease Control and Prevention. Atrial Fibrillation Fact Sheet. Available online: https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_atrial_fibrillation.htm
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5. [Crossref] [PubMed]
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071-104. [Crossref] [PubMed]
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131:e29-322. [Crossref] [PubMed]
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528-606. [Crossref] [PubMed]
- Shah A, Pedersen M, Hocini M. Recent insights into the mechanisms underlying persistent atrial fibrillation. J Innovations Cardiac Rhythm Management 2012;3:892-8.
- Iwasaki YK, Nishida K, Kato T, et al. Atrial fibrillation pathophysiology: implications for management. Circulation 2011;124:2264-74. [Crossref] [PubMed]
- Verma A, Macle L, Cox J, et al. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: catheter ablation for atrial fibrillation/atrial flutter. Can J Cardiol 2011;27:60-6. [Crossref] [PubMed]
- Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J 1959;58:59-70. [Crossref] [PubMed]
- Narayan SM, Patel J, Mulpuru S, et al. Focal impulse and rotor modulation ablation of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on follow-up: A video case study. Heart Rhythm 2012;9:1436-9. [Crossref] [PubMed]
- Davidenko JM, Kent PF, Chialvo DR, et al. Sustained vortex-like waves in normal isolated ventricular muscle. Proc Natl Acad Sci U S A 1990;87:8785-9. [Crossref] [PubMed]
- Haïssaguerre M, Marcus FI, Fischer B, et al. Radiofrequency catheter ablation in unusual mechanisms of atrial fibrillation: report of three cases. J Cardiovasc Electrophysiol 1994;5:743-51. [Crossref] [PubMed]
- Ho SY, Sanchez-Quintana D, Cabrera JA, et al. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 1999;10:1525-33. [PubMed]
- Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins. An anatomic study of human hearts. Circulation 1966;34:412-22. [Crossref] [PubMed]
- Antzelevitch C, Burashnikov A. Overview of Basic Mechanisms of Cardiac Arrhythmia. Card Electrophysiol Clin 2011;3:23-45. [Crossref] [PubMed]
- Wakili R, Voigt N, Kääb S, et al. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 2011;121:2955-68. [Crossref] [PubMed]
- Arora R. Recent insights into the role of the autonomic nervous system in the creation of substrate for atrial fibrillation: implications for therapies targeting the atrial autonomic nervous system. Circ Arrhythm Electrophysiol 2012;5:850-9. [Crossref] [PubMed]
- Chen PS, Chen LS, Fishbein MC, et al. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 2014;114:1500-15. [Crossref] [PubMed]
- Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004;109:327-34. [Crossref] [PubMed]
- Katritsis DG, Pokushalov E, Romanov A, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol 2013;62:2318-25. [Crossref] [PubMed]
- Zheng S, Li Y, Han J, et al. Long-term results of a minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation. PLOS One 2013;8:1-8. [Crossref] [PubMed]
- Boldt A, Wetzel U, Lauschke J, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 2004;90:400-5. [Crossref] [PubMed]
- Platonov PG, Mitrofanova LB, Orshanskaya V, et al. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol 2011;58:2225-32. [Crossref] [PubMed]
- Kuppahally SS, Akoum N, Burgon NS, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 2010;3:231-9. [Crossref] [PubMed]
- Stiles MK, John B, Wong CX, et al. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the “second factor”. J Am Coll Cardiol 2009;53:1182-91. [Crossref] [PubMed]
- Teh AW, Kistler PM, Lee G, et al. Electroanatomic remodeling of the left atrium in paroxysmal and persistent atrial fibrillation patients without structural heart disease. J Cardiovasc Electrophysiol 2012;23:232-8. [Crossref] [PubMed]
- Pokharel S, van Geel PP, Sharma UC, et al. Increased myocardial collagen content in transgenic rats overexpressing cardiac angiotensin-converting enzyme is related to enhanced breakdown of N-acetyl-Ser-Asp-Lys-Pro and increased phosphorylation of Smad2/3. Circulation 2004;110:3129-35. [Crossref] [PubMed]
- Thomas MC, Dublin S, Kaplan RC, et al. Blood pressure control and risk of incident atrial fibrillation. Am J Hypertens 2008;21:1111-6. [Crossref] [PubMed]
- Medi C, Kalman JM, Spence SJ, et al. Atrial electrical and structural changes associated with longstanding hypertension in humans: implications for the substrate for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:1317-24. [Crossref] [PubMed]
- Mahnkopf C, Badger TJ, Burgon NS, et al. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: implications for disease progression and response to catheter ablation. Heart Rhythm 2010;7:1475-81. [Crossref] [PubMed]
- Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758-67. [Crossref] [PubMed]
- Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044-53. [Crossref] [PubMed]
- Konings KT, Smeets JL, Penn OC, et al. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation 1997;95:1231-41. [Crossref] [PubMed]
- Latchamsetty R, Morady F. Complex fractionated atrial electrograms: a worthwhile target for ablation of atrial fibrillation? Circ Arrhythm Electrophysiol 2011;4:117-8. [Crossref] [PubMed]
- Gerstenfeld EP, Lavi N, Bazan V, et al. Mechanism of complex fractionated electrograms recorded during atrial fibrillation in a canine model. Pacing Clin Electrophysiol 2011;34:844-57. [Crossref] [PubMed]
- Krummen DE, Swarup V, Narayan SM. The role of rotors in atrial fibrillation. J Thorac Dis 2015;7:142-51. [PubMed]
- Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res 2013;112:849-62. [Crossref] [PubMed]
- Nash MP, Mourad A, Clayton RH, et al. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation 2006;114:536-42. [Crossref] [PubMed]
- Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature 1998;392:75-8. [Crossref] [PubMed]
- Allessie M, de Groot N. CrossTalk opposing view: Rotors have not been demonstrated to be the drivers of atrial fibrillation. J Physiol 2014;592:3167-70. [Crossref] [PubMed]
- Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812-22. [Crossref] [PubMed]
- Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002;105:1077-81. [Crossref] [PubMed]
- Wilber DJ. Linear ablation for atrial fibrillation: have we come full circle?. J Am Coll Cardiol 2003;42:1283-5. [Crossref] [PubMed]
- Swartz JF, Perrersels G, Silvers J, et al. A catheter based curative approach to atrial fibrillation in humans Circulation 1994;90:I-335. (abstract).
- Ernst S, Schlüter M, Ouyang F, et al. Modification of the substrate for maintenance of idiopathic human atrial fibrillation: efficacy of radiofrequency ablation using nonfluoroscopic catheter guidance. Circulation 1999;100:2085-92. [Crossref] [PubMed]
- Ernst S, Ouyang F, Löber F, et al. Catheter-induced linear lesions in the left atrium in patients with atrial fibrillation: an electroanatomic study. J Am Coll Cardiol 2003;42:1271-82. [Crossref] [PubMed]
- Jaïs P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation 2004;110:2996-3002. [Crossref] [PubMed]
- Fassini G, Riva S, Chiodelli R, et al. Left mitral isthmus ablation associated with PV Isolation: long-term results of a prospective randomized study. J Cardiovasc Electrophysiol 2005;16:1150-6. [Crossref] [PubMed]
- Hocini M, Jaïs P, Sanders P, et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation 2005;112:3688-96. [Crossref] [PubMed]
- Wu L, Yao Y, Zheng L, et al. Long-term follow-up of pure linear ablation for persistent atrial fibrillation without circumferential pulmonary vein isolation. J Cardiovasc Electrophysiol 2014;25:471-6. [Crossref] [PubMed]
- Oral H, Chugh A, Good E, et al. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation 2007;115:2606-12. [Crossref] [PubMed]
- Oral H, Chugh A, Yoshida K, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol 2009;53:782-9. [Crossref] [PubMed]
- Verma A, Mantovan R, Macle L, et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J 2010;31:1344-56. [Crossref] [PubMed]
- Hayward RM, Upadhyay GA, Mela T, et al. Pulmonary vein isolation with complex fractionated atrial electrogram ablation for paroxysmal and nonparoxysmal atrial fibrillation: A meta-analysis. Heart Rhythm 2011;8:994-1000. [Crossref] [PubMed]
- Providência R, Lambiase PD, Srinivasan N, et al. Is There Still a Role for Complex Fractionated Atrial Electrogram Ablation in Addition to Pulmonary Vein Isolation in Patients With Paroxysmal and Persistent Atrial Fibrillation? Meta-Analysis of 1415 Patients. Circ Arrhythm Electrophysiol 2015;8:1017-29. [Crossref] [PubMed]
- Estner HL, Hessling G, Biegler R, et al. Complex fractionated atrial electrogram or linear ablation in patients with persistent atrial fibrillation--a prospective randomized study. Pacing Clin Electrophysiol 2011;34:939-48. [Crossref] [PubMed]
- Dixit S, Marchlinski FE, Lin D, et al. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol 2012;5:287-94. [Crossref] [PubMed]
- Kim TH, Uhm JS, Kim JY, et al. Does Additional Electrogram-Guided Ablation After Linear Ablation Reduce Recurrence After Catheter Ablation for Longstanding Persistent Atrial Fibrillation? A Prospective Randomized Study. J Am Heart Assoc 2017;6:e004811. [Crossref] [PubMed]
- Kabra R, Singh JP. Catheter ablation targeting complex fractionated atrial electrograms for the control of atrial fibrillation. Curr Opin Cardiol 2012;27:49-54. [Crossref] [PubMed]
- Narayan SM, Krummen DE, Donsky A, et al. Treatment of paroxysmal atrial fibrillation by targeted elimination of stable rotors and focal sources without pulmonary vein isolation: The precise rotor elimination without concomitant pulmonary vein isolation for subsequent elimination of PAF (PRECISE). Heart Rhythm 2013;10:LB10.
- Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628-36. [Crossref] [PubMed]
- Narayan SM, Baykaner T, Clopton P, et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol 2014;63:1761-8. [Crossref] [PubMed]
- Miller JM, Kowal RC, Swarup V, et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol 2014;25:921-9. [Crossref] [PubMed]
- Spitzer SG, Károlyi L, Rämmler C, et al. Treatment of recurrent nonparoxysmal atrial fibrillation using focal impulse and rotor mapping (FIRM)-guided rotor ablation: Early recurrence and long-term outcomes. J Cardiovasc Electrophysiol 2017;28:31-8. [Crossref] [PubMed]
- Miller JM, Kalra V, Das MK, et al. Clinical Benefit of Ablating Localized Sources for Human Atrial Fibrillation: The Indiana University FIRM Registry. J Am Coll Cardiol 2017;69:1247-56. [Crossref] [PubMed]
- Buch E, Share M, Tung R, et al. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: A multicenter experience. Heart Rhythm 2016;13:636-41. [Crossref] [PubMed]
- Steinberg JS, Shah Y, Bhatt A, et al. Focal impulse and rotor modulation: Acute procedural observations and extended clinical follow-up. Heart Rhythm 2017;14:192-7. [Crossref] [PubMed]
- Gianni C, Mohanty S, Di Biase L, et al. Acute and early outcomes of focal impulse and rotor modulation (FIRM)-guided rotors-only ablation in patients with nonparoxysmal atrial fibrillation. Heart Rhythm 2016;13:830-5. [Crossref] [PubMed]
- Allessie MA. Atrial electrophysiologic remodeling: another vicious circle? J Cardiovasc Electrophysiol 1998;9:1378-93. [Crossref] [PubMed]
- Akoum N, Daccarett M, McGann C, et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol 2011;22:16-22. [Crossref] [PubMed]
- Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498-506. [Crossref] [PubMed]
- Rolf S, Kircher S, Arya A, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:825-33. [Crossref] [PubMed]
- Cutler MJ, Johnson J, Abozguia K, et al. Impact of Voltage Mapping to Guide Whether to Perform Ablation of the Posterior Wall in Patients With Persistent Atrial Fibrillation. J Cardiovasc Electrophysiol 2016;27:13-21. [Crossref] [PubMed]
- Yamaguchi T, Tsuchiya T, Nakahara S, et al. Efficacy of Left Atrial Voltage-Based Catheter Ablation of Persistent Atrial Fibrillation. J Cardiovasc Electrophysiol 2016;27:1055-63. [Crossref] [PubMed]
- Yang G, Yang B, Wei Y, et al. Catheter Ablation of Nonparoxysmal Atrial Fibrillation Using Electrophysiologically Guided Substrate Modification During Sinus Rhythm After Pulmonary Vein Isolation. Circ Arrhythm Electrophysiol 2016;9:e003382. [Crossref] [PubMed]
- Nery PB, Thornhill R, Nair GM, et al. Scar-based catheter ablation for persistent atrial fibrillation. Curr Opin Cardiol 2017;32:1-9. [Crossref] [PubMed]
- Kottkamp H, Berg J, Bender R, et al. Box Isolation of Fibrotic Areas (BIFA): A Patient-Tailored Substrate Modification Approach for Ablation of Atrial Fibrillation. J Cardiovasc Electrophysiol 2016;27:22-30. [Crossref] [PubMed]
- Kottkamp H, Bender R, Berg J. Catheter ablation of atrial fibrillation: how to modify the substrate? J Am Coll Cardiol 2015;65:196-206. [Crossref] [PubMed]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation 2010;122:109-18. [Crossref] [PubMed]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation. J Am Coll Cardiol 2016;68:1929-40. [Crossref] [PubMed]
- Lakkireddy D, Sridhar Mahankali A, Kanmanthareddy A, et al. Left atrial appendage ligation and ablation for persistent atrial fibrillation: The LAALA-AF registry. JACC Clin Electrophysiol 2015;1:153-60. [Crossref]
- Cox JL, Schuessler RB, Boineau JP. The development of the Maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg 2000;12:2-14. [Crossref] [PubMed]
- Damiano RJ Jr, Schwartz FH, Bailey MS, et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg 2011;141:113-21. [Crossref] [PubMed]
- Edgerton JR, Edgerton ZJ, Weaver T, et al. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. Ann Thorac Surg 2008;86:35-8; discussion 39. [Crossref] [PubMed]
- Zembala M, Filipiak K, Kowalski O, et al. Staged hybrid ablation for persistent and longstanding persistent atrial fibrillation effectively restores sinus rhythm in long-term observation. Arch Med Sci 2017;13:109-17. [Crossref] [PubMed]
- Geršak B, Zembala MO, Müller D, et al. European experience of the convergent atrial fibrillation procedure: multicenter outcomes in consecutive patients. J Thorac Cardiovasc Surg 2014;147:1411-6. [Crossref] [PubMed]
- Geršak B, Jan M. Long-Term Success for the Convergent Atrial Fibrillation Procedure: 4-Year Outcomes. Ann Thorac Surg 2016;102:1550-7. [Crossref] [PubMed]
- Bisleri G, Rosati F, Bontempi L, et al. Hybrid approach for the treatment of long-standing persistent atrial fibrillation: electrophysiological findings and clinical results. Eur J Cardiothorac Surg 2013;44:919-23. [Crossref] [PubMed]


