Prospective study of quality of life after lung cancer resection
Introduction
In 2016, it was estimated that newly diagnosed cases of lung and bronchus cancer would exceed 224,000 (1). Surgical resection with curative-intent remains the gold standard for patients with clinically operable early-stage non-small cell lung cancer (NSCLC) (2). Since 2010, over 82,000 pneumonectomies or lobectomies are performed annually in the United States (3). These increases in technical safety and early recovery have made thoracoscopic surgery and video assisted thoracic surgery (VATS) more popular (4).
Chronic pain following thoracic procedures has long been identified (5). This recognized dysfunction is labeled “Post Thoracotomy Pain Syndrome (PTPS)” and is defined as pain localized to the incision scar or the intercostal dermatome distribution that persists beyond 2 months post-operatively (6). Various methods have been recommended to minimize the risk of post thoracotomy pain such as the use of muscle sparing thoracotomy, avoiding rib spreading, and placing closure sutures through the ribs (7). We have shown early post-surgical benefits to VATS versus thoracotomy with respect to early postoperative pain and shoulder function; however there were no long term differences (8,9). In clinical investigation of PTPS researchers noted mood disorders such as anxiety and depression may impact patient outcomes including acceptance of treatment, pain tolerance, and quality of life (QOL) (10). Therefore a clinical assessment of mood disorders adds value to clinical investigations (11). Post-operative life style changes include: physical, social/family, emotional, and pulmonary function, which often impact patients’ QOL. Measuring the impact of treatment on QOL has been well documented in the evaluation of therapeutic effectiveness and therefore should be included in clinical studies (12).
The primary goal of this study was to investigate differences with respect to demographics, chronic pain, mood disorders and QOL between the patients who had VATS versus thoracotomy for lung cancer resection. The secondary goals were to investigate whether there were differences between the thoracotomy patients who had conversions from VATS versus those had initial thoracotomy.
Methods
Patients were selected between 2010 and 2014 from 3 of the 14 hospitals at a university medical center. All patients were between 40 and 85 years of age with potentially resectable lung cancer (Stage I–IIIA), without dementia, able to understand English, and completed two questionnaires between 2 months and 1 year after surgery. The study was approved by the University Institutional Review Board and all participants provided written informed consent. The choice of surgical approach was dictated by surgeon preference.
Pre-operative evaluation included routine cardio-pulmonary review with spirometry and if indicated a diffusion capacity. Nuclear cardiac studies were generally, but not universally performed. CT scans of the chest and abdomen along with PET scans were standard.
The lateral decubitus position is typical for either thoracotomy or VATS. Standard thoracotomy is performed with division of the latissimus muscle and preservation of the serratus muscle. An interspace incision is performed usually with resection of a small portion of the lower rib posteriorly. A rib spreading retractor is used.
A VATS procedure is common in our practice and is considered to be minimally invasive because the approach does not involve rib-spreading and only requires 3 to 5 small, 5 to 10 millimeter, incisions strategically placed to permit insertion of the thoracoscope, and working instruments through appropriate sized ports. However, even in this procedure nerve injuries and rib bruising or fractures may occur from trocar insertion or torqueing (13,14).
In some cases, particularly early in this series the initial VATS procedure needed to be converted to a thoracotomy (13,15). These patients are included in the thoracotomy group. Thus many of the more complex procedures (lobectomy etc.) had thoracotomies, while the less complex procedures (wedge resections) were performed by VATS. Additionally, VATS procedures usually require the use of an access or utility incision (typically 5 cm) to allow use of instruments and extraction of the specimen. For the utility incision a wound protector is standard, but a rib spreader is not used (13).
Data collection and instruments used to measure QOL
Medical records were used to identify: cancer stage, surgical treatment, and medical interventions for pain. Participants completed a 0–10 [numerical assessment scale (NAS)] pain assessment and two questionnaires. The questionnaire for mood disorders was the Hospital Anxiety and Depression Scale (HADS) and for QOL was Functional Assessment of Cancer Therapy-Lung (FACT-L).
The HADS questionnaire is a 14 item, self-assessment tool developed to measure patient’s mood disorders with two sub scales: anxiety and depression. Each sub scale is composed of seven questions using a 0 to 3 Likert scale. Mood is evaluated using the sum of each sub scale score: 0 to 7 normal, 8 to 10 borderline and 11 to 21 abnormal. The medical records of all patients with sub score values greater than 11 were checked for treatment (11).
FACT-L is a 44 item, 5 sub scale self-assessment tool developed to measure a lung cancer patient’s QOL in the last 7 days, with each sub scale using a 0 to 4 Likert scale. The four sub scales (emotional, functional, physical, and social) query patient’s well-being. The fifth sub scale queries the patient’s pulmonary (lung) functionality (16).
All instruments total scores were the sum of their sub scales and have historically been reported as reliable and valid (11,16). These measures took an average of 5 minutes each to complete, with the option to complete the questionnaires in clinic or at home and return them in a pre-stamped envelope to the principle investigator.
Statistical analysis
SPSS (Version 22, IBM-SPSS, Inc., Armonk, New York, USA) was used in the analysis of the differences between thoracotomy and VATS patients with respect to demographics, chronic pain (NAS), mood disorders (HADS) and QOL (FACT-L). Comparisons between participant’s surgical techniques were made using Chi-Square test, as indicated in the tables. The Mann Whitney test was used to test for statistical significance because the responses were not normally distributed. Comparisons between the subcategories >2 used the Kruskal-Wallis test. When significant differences were found, post-hoc comparisons were performed to detect the point of difference. Statistical significance was set at P≤0.05 for all variables.
Results
The major findings of this study were Table 1: (I) there were no statistically significant differences between the thoracotomy or VATS groups in demographics, chronic pain, mood disorders or QOL; (II) based upon medical records the surgical approach of choice was a thoracotomy for patients with stage IIIa neoplasms, pneumonectomy or a bi-lobectomy; (III) 22 of the 66 (33.3%) thoracotomy patients were converted from VATS; and (IV) regardless of the surgical approach the majority of all participants had relatively low intensity of chronic pain, low to moderate anxiety and depression, with moderate to high QOL.

Full table
A total of 110 patients were consented, but only 97 completed the study. The surgical approach for lung cancer resection in these patients was a thoracotomy (N=66) in 68% or a VATS approach in (N=31) 32% of patients. Missing data were confined to two of the 97 subjects who did not return one or more questionnaires; one subject did not return the mood disorders (HADS total score) and a second subject did not return the QOL (FACT-L questionnaire).
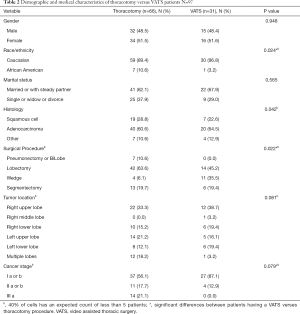
In reviewing the patient socio-economic based demographics, there were no statistical or clinical differences between these two surgical approaches with respect to age (P=0.446), pack years (P=0.547), education (P=0.665), time since surgery (P=0.697), gender (P=0.948) and marital status (P=0.565) (Tables 1,2). The majority of our population was: 65 years or older (62.9%), smoked (81.4%), and about half (50.5%) were out at least 6 months post-operative. Gender was evenly distributed between both thoracotomy (32 males and 34 females) and VATS (15 males and 16 females). There were differences between the two surgical approaches with respect to histology (P=0.042) and race (P=0.24). The majority (61.9%) of patients had adenocarcinoma.
Full table
There was no statistical significance in the surgical approach when analyzed as to the tumor lobe location (P=0.081). Statistical significance was approached with tumor stage (P=0.079). Thoracotomy was performed for all of stage IIIa neoplasms; while for earlier stage neoplasms (Ia or Ib) VATS resection was often performed (87.1% vs. 56.1%). Pneumonectomy and bi-lobectomies were only performed by a thoracotomy. Of the 34 sub-lobar resections 17 were performed by thoracotomy and 17 by VATS. More specifically, single lobectomies were more likely to have a VATS than thoracotomy resection (63.6% vs. 45.2%). Wedge resections were predominately performed via VATS resection (35.5% vs. 6.1%). Segmental resections were more evenly distributed between thoracotomy and VATS resection (19.7% vs. 19.4%).
QOL: thoracotomy vs. VATS resection comparisons
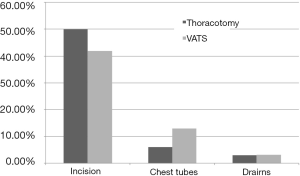
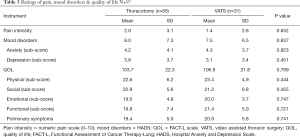
We compared the outcomes of thoracotomy vs. VATS resections with respect to pain, the association of mood disorders (HADS total score), and QOL (FACT total score). Pain was evaluated by the NAS of 1 to 10. The site of pain reported was at the main incision site (n=49, 50.1%), site of the chest tube (CT) (n=8, 11%) and site of secondary drains (n=4, 4.1%) (Figure 1). There were no statistically significant differences between the thoracotomy (n=66) and VATS (n=31) resection groups with respect to level of chronic post-operative pain (P=0.652) (Table 3, Figure 2). Thoracotomy and VATS patients had a mean pain level of 2.0 SD ±3.1 and 1.4 SD ±2.6, respectively. The pain levels ranged from 0–10, with the majority (95%) having mild to moderate pain (range <7). Less than 5% reported severe pain (greater than 7) and were referred to pain clinic.
Full table
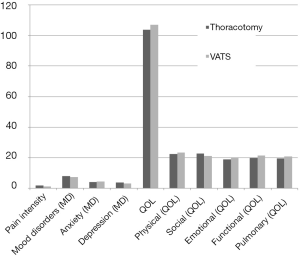
There was also no statistical difference between the two surgical approaches when comparing mood disorders and QOL total scores, P=0.837 and P=0.799, respectively. The mean score for mood disorders compared for patients with thoracotomy and VATS resections were 8.0 SD ±7.3 and 7.5 SD ±6.5, respectively (P=0.837). Similarly there was no statistical difference between the surgical approaches for each of the mood disorder subscales anxiety (P=0.823) and depression (P=0.451). The majority (91%), of all post-surgical patients, had mild to moderate mood disorders. Less than 9% showed some level of a severe mood disorder and all 9% were under some form of medical treatment. With respect to QOL both post-surgical groups had minimal QOL issues; with the majority (98%) having no QOL issues. We noted VATS resection patients having a slightly higher mean (slightly better QOL) of (106.9 SD ±21.8) compared to (103.7 SD ±22.3) for thoracotomy, but this was not significant (P=0.799). Additionally, there was no statistical difference between the surgical approach for each of the QOL subscales: physical (P=0.444), social (P=0.455), functional (P=0.747), emotional (P=0.721); and pulmonary (P=0.741).
Insights into VATS to thoracotomy conversions
The secondary goal was to investigate differences between patients converted from VATS to thoracotomy verses those managed initially with thoracotomy. VATS conversions as defined in this paper require thoracotomy and the use of rib-spreading after the VATS resection has begun. We compared those patients who had initial thoracotomy to those who had VATS conversions with respect to chronic pain, mood disorders and QOL (Table 4, Figure 3). There were no statistically significant differences between the groups. Initial thoracotomy patients had a mean pain level of (1.9 SD ±3.0) and conversion patients’ mean (2.2 SD ±3.3). Three to five percent of patients in both groups reported severe chronic pain and were referred to pain clinic.
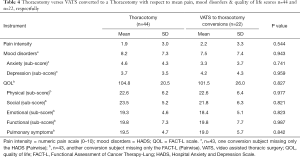
Full table
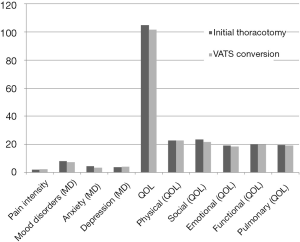
There was no statistical difference between the two surgical approaches when comparing mood disorder and QOL total scores, (P=0.943 and P=0.959 respectively). Eight percent showed some levels of a mood disorder. They were already under some form of medical treatment.
Both sets of patients reported high mean QOL total scores (104.8 SD ±20.5 and 101.5 SD ±26.0, respectively). Similarly there was no statistical difference between the surgical approach for each of the well-being subscales: physical (P=0.977), social (P=0.821), functional (P=0.823), emotional (P=0.987); and pulmonary (P=0.842). The majority (99%) having no QOL issues and relatively high QOL.
Discussion
In an era with increasing medical innovation in the area of surgical intervention, the assessment of outcomes and efficacy of VATS procedures (robotic and non-robotic) is increasingly relevant to thoracic surgeons and their patients. It has been reported that there was a nine-fold increase in the utilization of VATS from the mid 90’s the early 21st century and this trend appears to be increasing as the skill and experience at high volume institutions continues to increase (17). Prior series have shown VATS to be equivalent or better than conventional thoracotomy in terms of operative morbidity and mortality (4). As literature continues to accumulate comparing VATS and thoracotomy on the basis of surgical outcomes, there remains a lack of prospective trials comparing these two operative techniques on the basis of chronic post-operative pain and QOL indicators.
A multicenter analysis was performed with 165 thoracotomy patients compared to 178 VATS. The operative groups (Thoracotomy vs. VATS) were further stratified into two cohorts: (I) patients responses to the questionnaire less than 1 year postoperatively; (II) responses greater than 1 year postoperatively. In the <1 year cohort, patients who had VATS reported less pain and shoulder dysfunction than those who had thoracotomy; although, pain medication requirements were similar. There were no significant differences in pain-related parameters for patients after 1 year (8).
The prospective studies comparing VATS and thoracotomy in the first post-operative year with respect to pain, anxiety depression, and QOL are relatively limited. Wildgaard and colleagues in a study of 546 patients evaluated postoperative pain in VATS and thoracotomy patients. Their series included an average follow-up of 22 months (range 12–36 months). There was pain reported in 25% of VATS patients and 33% of thoracotomy patients. There were no significant differences in comparisons of VATS to thoracotomy in regards to: prevalence, distribution of pain, sensory changes, and effect of pain on daily activities (18).
Stammnerger presented favorable results in regards to chronic post-operative pain from VATS. This study however was not a comparative study evaluating pain after VATS vs. thoracotomy, and only included patients treated with a VATS approach. In addition, this series included patients with benign lesions, in contrast to our current study. Pain questionaires were given to 213 patients who underwent VATS for various conditions including: pneumothorax, solitary pulmonary nodules, pleural effusion and 173 patients returned responses with a mean follow-up of 18 [3–38] months. At 6 months post-operatively 75% of patients had no complaints; 20% had diffuse chest discomfort; and 5% had scar pain. At 1-year post-op 86% of patients had no complaints, 9% experienced minimal pain, and 5% had moderate pain. Two years post-op 96% of patients had no complaints (19).
In contrast to the relatively limited incidence of pain presented in the previous study, Mongardon and colleagues presented the results of a study including 86 patients who received a pain questionnaire 1 year after posterolateral thoracotomy. Of the 65 patients who responded, (n=31, 48%) patients reported chronic pain. The chronic pain group was approximately 10 years younger than those without pain. Patients reported pain in various locations with the majority at the scar (28%) (19). This study again only included the thoracotomy approach, and did not include patients with a VATS approach.
Prospective QOL studies comparing VATS and thoracotomy with respect to post-operative functionality are limited. Our study’s results were similar to a smaller study done by Wilson et al. 2008. They performed an analysis including 51 patients who underwent VATS (n=27) or thoracotomy (n=24) for NSCLC using a Chinese version of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30 and the EORTC QLQ-LC13. The incidence of symptoms were considered high in both groups, and QOL scores presented high levels of functionality in both surgical groups of patients (20). This study also noted a trend to score higher on QOL and functionality for VATS but these differences were not statistically significant.
Strengths and limitations
This is one of the largest studies comparing chronic pain and QOL in VATS vs. thoracotomy approach. In addition, in contrast to some previous studies, in order to decrease the heterogeneity of the study population, we have focused on patients who had surgery for cancer, and did not include patients who underwent surgery for benign conditions. Further, another strength of our study, is the use of validated instruments to evaluate the QOL. Our study does have limitations. This study is not a prospective randomized study, and has limitations including selection bias, that is, the treating physician decided on the surgical approach. In addition, the patient cohort although limited to those with lung cancer, is heterogeneous comprising of various stages of cancer, and groups are not completely balanced with more advanced stage patients treated with an open approach. One of the other limitations of the prospective cross sectional design used in our study is that our assessment of pain, mood disorders and QOL were limited to a single time point. It is possible that these variables may have differed over time. However, we found no difference in the number of patients reporting symptoms based on time since surgery. This patient cohort was recruited from a high volume academic service specializing in thoracic surgery, and therefore these results may not be generalizable to other practice settings. The majority of participants were Caucasians and slightly more Caucasians had VATS procedures while African Americans were more likely to have thoracotomies. Thus, this study may not be applicable in more ethnically diverse regions. Finally, we did not distinguish between muscle sparing and open thoracotomy. Despite these limitations, this study is one of the largest studies with a prospective study design reported in the literature evaluating the QOL comparing a VATS approach to a thoracotomy
Conclusions
In this prospective study of lung cancer patients, we evaluated the longer term QOL comparing a VATS approach to thoracotomy approach for lung resection. While previous studies have shown that a VATS approach offers an early advantage with regards to perioperative outcomes, our study showed that VATS and thoracotomy patients had a similar late QOL outcomes. Further prospective studies are required to fully evaluate the QOL outcomes between these approaches.
Acknowledgements
Funding: Kathleen G. Hopkins (PhD, RN) was funded by the National Institute of Nursing Research (F31 NR0131114), Nursing Foundation of Pennsylvania (Pauline Thompson Clinical Nursing Research Award), and the Oncology Nursing Foundation, Oncology Nursing Society (Research Grant).
Arjun Pennathur (MD, FACS) was funded by National Institutes of Health (NIH) Specialized Program of Research Excellence (SPORE) in Lung Cancer (P50 CA090440), National Institute of Nursing Research (F31 NR0131114), Nursing Foundation of Pennsylvania (Pauline Thompson Clinical Nursing Research Award), and the Oncology Nursing Foundation, Oncology Nursing Society (Research Grant) and the Sampson Family Endowed Chair, University of Pittsburgh.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the University Institutional Review Board and all participants provided written informed consent.
References
- American Cancer Society. Cancer Facts & Figures 2016. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Non-Small Cell Lung Cancer V.3.2017. Available online: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Weiss AJ, Elixhauser A, Steiner C. Readmissions to U.S. Hospitals by Procedure, 2010. Available online: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb154.pdf
- Whitson BA, Andrade RS, Boettcher A, et al. Video-Assisted Thoracoscopic Surgery is More Favorable Than Thoracotomy for Resection of Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Blades B, Dugan DJ. War wounds of the chest observed at the Thoracic Surgery Center. Walter Reed General Hospital. J Thorac Surg 1944;13:294-306.
- Merskey H, Bogduk H. Classification of chronic pain. Second ed. Descriptions of chronic pain syndromes and definitions of pain terms. Seattle, WA: IASP Press;1994.
- Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg 2009;36:170-80. [Crossref] [PubMed]
- Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J Thorac Cardiovasc Surg 1994;107:1079-85; discussion 85-6. [PubMed]
- Landreneau RJ, Wiechmann RJ, Hazelrigg SR, et al. Effect of minimally invasive thoracic surgical approaches on acute and chronic postoperative pain. Chest Surg Clin N Am 1998;8:891-906. [PubMed]
- Cheng KK, Lee DT. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol 2011;78:127-37. [Crossref] [PubMed]
- Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes 2003;1:29. [Crossref] [PubMed]
- Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol 2002;55:285-95. [Crossref] [PubMed]
- Demmy TL, James TA, Swanson SJ, et al. Troubleshooting video-assisted thoracic surgery lobectomy. Ann Thorac Surg 2005;79:1744-52; discussion 53.
- Rogers ML, Duffy JP. Surgical aspects of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 2000;18:711-6. [Crossref] [PubMed]
- Aoki T, Tsuchida M, Hashimoto T, et al. Quality of life after lung surgery: Video-assisted thoracic surgery verses thoracotomy. Heart Lung Circ 2007;16:285-9. [Crossref] [PubMed]
- Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220. [Crossref] [PubMed]
- Farjah F, Wood DE, Mulligan MS, et al. Safety and efficacy of video-assisted versus conventional lung resection for lung cancer. J Thorac Cardiovasc Surg 2009;137:1415-21. [Crossref] [PubMed]
- Wildgaard K, Ravn J, Nikolajsen L, et al. Consequences of persistent pain after lung cancer surgery: a nationwide questionnaire study. Acta Anaesthesiol Scand 2011;55:60-8. [Crossref] [PubMed]
- Mongardon N, Pinton-Gonnet C, Szekely B, et al. Assessment of Chronic Pain After Thoracotomy A 1-Year Prevalence Study. Clin J Pain 2011;27:677-81. [Crossref] [PubMed]
- Wilson CM, Tobin S, Young RC. The exploding worldwide cancer burden: the impact of cancer on women. Int J Gynecol Cancer 2004;14:1-11. [Crossref] [PubMed]


