Hairy cell leukaemia variant with periarticular joint infiltration and excellent radiotherapy response
Introduction
Hairy cell leukaemia (HCL) is rare, accounting for only 2% of leukaemias (1). An even more infrequent variant has been described, HCL-V (2,3). In this report, we present the case of a man with HCL-V diagnosed 12 years previously, who is currently haematologically stable with an unusual complication of joint pain due to extensive bony expansion secondary to leukaemic infiltration, and atypical skeletal imaging. His painful joint disease responded dramatically to radiotherapy.
Case presentation
A 73-year-old Caucasian male was diagnosed with HCL-V and underwent therapeutic splenectomy. He received no other form of systemic treatment at this stage. Nine years later the patient began to experience intermittent joint pain, the cause of which was unclear clinically. He was referred to a rheumatologist who excluded a chronic inflammatory arthropathy, osteoarthritis, and gout. Plain radiology showed changes in numerous bones consistent with bony erosion secondary to his chronic leukaemia. The patient had a bone scan which had a very unusual appearance, with increased radiotracer activity demonstrated within and adjacent to the majority of his large joints (Figure 1). The activity demonstrated within many of these joints was consistent with inflammatory arthritis at these sites; however, uptake around the joints was atypical and likely to reflect bony expansion related to abnormal haematopoeisis.
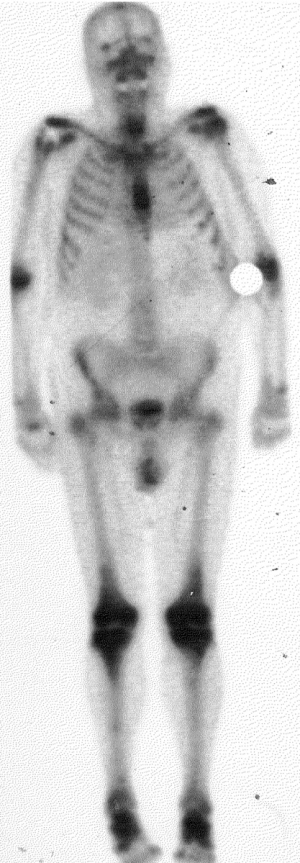
The primary location of pain was his left leg, in the region of the knee joint. CT and MRI demonstrated extensive marrow infiltration involving the lower femur, upper tibia, the neck of the fibula, and the patella with evidence of cortical destruction. A biopsy to confirm that this was malignant leukaemic expansion was not performed due to the significant risk of a complicating stress fracture. The patient was referred for palliative radiotherapy. He received 20 Gy in five 4 Gy fractions using anterio-posterior 6 MeV photon fields to his left lower femur and left upper tibia/fibula. The patient had a very rapid symptomatic response to the radiotherapy, with pain resolving the day following his first fraction, without a radiotherapy flare reaction. Following this treatment, he developed discomfort in his right elbow. Plain radiographs demonstrated significant bony erosion in the distal humerus, proximal radius and ulna, and an infiltrative process similar to that in the left knee on CT. He received a further 20 Gy in five fractions to his right elbow with excellent symptomatic response once again.
The patient is being followed up regularly and 12 years post diagnosis he is still alive with excellent bone marrow function.
Discussion
HCL, a B-cell lymphoproliferative disorder, is rare, constituting approximately 2% of all cases of leukaemia and is characterised by an indolent course, peripheral cytopenias and splenomegaly (1). The diagnosis of HCL is based upon recognition of neoplastic lymphocytes with external cytoplasmic projections (“hairs”) in peripheral blood smears and a typical pattern of infiltration in bone marrow biopsies, with reticulin fibrosis (1). Other diagnostic features include raised levels of soluble interleukin 2 receptors (solIL-2L) and positivity for tartrate resistant acid phosphatase (TRAP) (4).
In 1980 a variant form of this disease was first described by Cawley et al. (5) and a number of case reports have been since published, some designating this condition a prolymphocytic variant of HCL. HCL-V is a very rare condition, accounting for <10% of HCL cases and sharing many clinical, morphologic, and immunophenotypic features with the classical form of the disease (2,3), as summarized and compared and contrasted with the features of HCL, in Table 1. The major clinical features of HCL-V include lymphocytosis, cytopenias without monocytopenia and splenomegaly (2,3,6). Gross splenomegaly is present in approximately 85% of HCL-V patients and splenectomy has been shown to provide good palliation resulting in long-lasting partial responses in over two-thirds of patients from 1–10+ years, with a median of 4 years (2,7). Given the exquisite radiosensitivity of HCL/HCL-V, therapeutic splenic irradiation should also be considered as an option for significant splenomegaly.
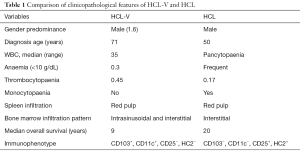
Full table
Morphologically, the neoplastic cells appear to be intermediates between prolymphocytes and characteristic “hairy” neoplastic lymphocytes of HCL. The immunophenotype is that of a mature B-cell with positivity for CD103 and CD11c B-cell antigens; however in contrast to HCL, these cells are typically negative for CD25 and HC2 (8). Histologically, the pattern of infiltration in the bone marrow and spleen is similar to HCL (7). Unlike HCL, patients with the variant form typically have “easy-to-aspirate bone marrow” and diminished response to conventional therapies such as interferon alpha and cladribine (6,9,10).
Haematologically, our patient was not anaemic or thrombocytopaenic and had white blood cell counts ranging between 13.4×109 and 20.6×109/L over the last 5 years of his illness. Anaemia and thrombocytopaenia are present in approximately one-third of HCL-V patients; however this is usually due to hypersplenism rather than bone marrow failure, as bone marrow lymphoid infiltration is usually mild. Our patient, over 11 years since splenectomy, was haematologically stable with good marrow function; achieving a better than average clinical response, and surviving longer than the average patient with HCL-V. Interestingly, while our patient has had a splenectomy, his bone pain and subsequent imaging have demonstrated extensive marrow infiltration in various bones, yet he remains haematologically stable. In addition, his current WBC count remains lower than the average of 34×109/L (range, 4×109–346×109/L) reported by Matutes et al. in 2003 (3).
Our patient’s main pathology related to skeletal manifestations of the disease. This is a very unusual manifestation of classic HCL (11). Although no such data exist for HCL-V, they are informative in relation to our patient. Of these unusual manifestations, skeletal osteolytic lesions were found to occur in only approximately 3% of patients. There have been reports of lytic lesions in the axial skeleton and long bones as well as the hip joint. In addition, osteoporosis, focal osteoblastic changes or sclerosis, and mixed patterns have been seen in HCL-V; bone pain was described as a rare complication of HCL (2,3). Imaging patterns of periarticular uptake have been described in haemoproliferative disorders and probably reflect bony expansion related to abnormal haemopoeisis (12).
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The patient has given his consent for us to publish this de-identified image of his bone scan.
References
- Foucar K, Catovsky D. Hairy cell leukaemia: WHO Classification of Haematopoietic and Lymphoid Tumours. Lyon: IARC Press, 2001:138-41.
- Matutes E, Martínez-Trillos A, Campo E. Hairy cell leukaemia-variant: Disease features and treatment. Best Pract Res Clin Haematol 2015;28:253-63. [Crossref] [PubMed]
- Matutes E, Wotherspoon A, Catovsky D. The variant form of hairy-cell leukaemia. Best Pract Res Clin Haematol 2003;16:41-56. [Crossref] [PubMed]
- Polliac A. Hairy Cell Leukemia: Biology, Clinical Diagnosis, Unusual Manifestations, and Associated Disorders. Rev Clin Exp Hematol 2002;6:366-88. [Crossref] [PubMed]
- Cawley JC, Burns GF, Hayhoe FG. A chronic lymphproliferative disorder with distinctive features: a distinct variant of hairy cell leukemia. Leuk Res 1980;4:547-59. [Crossref] [PubMed]
- Cessna MH, Hartung L, Tripp S, et al. Hairy Cell Leukemia Variant. Am J Clin Path 2005;123:132-8. [Crossref] [PubMed]
- Matutes E, Wotherspoon A, Brito-Babapulle V, et al. The natural history and clinico-pathological features of the variant form of hairy cell leukemia. Leukemia 2001;15:184-6. [Crossref] [PubMed]
- Matutes E, Morilla R, Owusu-Ankomah K, et al. The immunophenotype of hairy cell leukemia (HCL). Leuk Lymphoma 1994;14:57-61. [PubMed]
- Sainati L, Matutes E, Mulligan S, et al. A variant form of hairy cell leukemia resistant to alpha-interferon: clinical and phenotypic characteristics of 17 patients. Blood 1990;76:157-62. [PubMed]
- Tetreault SA, Robbins BA, Saven A. Treatment of hairy cell leukemia-variant with cladribine. Leuk Lymphoma 1999;35:347-54. [Crossref] [PubMed]
- Bouroncle BA. Unusual presentations and complications of hairy cell leukemia. Leukemia 1987;1:288-93. [PubMed]
- Evans TI, Nercessian BM, Sanders KM. Leukemic arthritis. Semin Arthritis Rheum 1994;24:48-56. [Crossref] [PubMed]


