Localized irradiation of mouse legs using an image-guided robotic linear accelerator
Introduction
The implantation of immortalized human muscle satellite cells into immunodeficient mice has been established to study muscle regeneration (1). One method to inhibit the regeneration capacity at the implantation site of muscle fibers with active stem-cells is the irradiation of the host muscle. In mice the anterior tibial muscle can serve as a host for muscle fiber implantation. To inactivate the regeneration capacity photon irradiation has been successfully used in previous trials (2,3). The application of high radiation doses with high precision and accuracy in order to restrict radiation and therefore side effects to small animals like mice might be difficult, because conventional irradiation systems are usually not designed to apply high doses with sub millimeter precision.
Although several irradiation devices designed to irradiate small animals with kV beams exist and are commercially available, these platforms are only valuable in high volume centers or when very high precision is required. Since none of such specialized platforms was available the investigators tried to establish a simple but yet precise irradiation for their needs with available linear accelerators in a radio oncologic department (4).
Here we report our experience using a dedicated radiosurgery system with integrated image guidance to apply a high single irradiation dose with high precision and accuracy to the anterior tibial muscle of mice.
Methods
Animals
All experiments were performed with the approval of the Landesamt für Gesundheit und Soziales, Berlin, according to § 8 Abs. 1 of the German animal protection law.
Seven weeks old female C57BL/6 mice (Charles River Laboratories, Sulzberg, Germany) have been used for the preliminary test series and immune deficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (EPO Berlin-Buch, GmbH, Berlin, Germany) have been used for the transplantation group.
One half of the immune deficient animals was treated with cardiotoxin injections into the anterior tibial muscle in order to destroy the muscle tissue and monitor the regeneration capability thereafter.
Anesthesia
All animals were anesthetized with Ketamine-Rompun (Ketamine 9 mg/mL, Rompun 1.2 mg/mL) with a dose of 160 µL/20 g administered by intraperitoneal injection.
Positioning
To provide a reproducible positioning of the mice during CT scan and treatment an acrylic glass block with surface fiducial markers was constructed. One leg of the mouse was pulled into the rectangular canal of the acrylic block with a rubber band, so the anterior tibial muscle was exposed in the acrylic canal and the mouse's body was somewhat kept away from the leg (Figure 1).
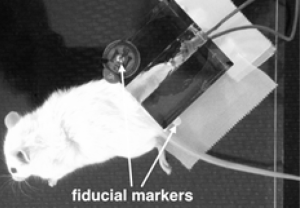
For the calculation of positioning radiographs as well as for treatment planning a computed tomography (CT) scan with 0.75 mm slice thickness of one of the healthy mice was conducted under intraperitoneal anesthesia using a conventional 16 slices CT scanner (Siemens Emotion, Erlangen, Germany). This CT dataset served as basis for tracking calculations as well as radiation dose distribution, assuming that all targets of the selected mice were somewhat similar. Since all mice were put in the same fixation position and tracking was focused on the acrylic block, individual CT scans could be renounced.
Treatment planning
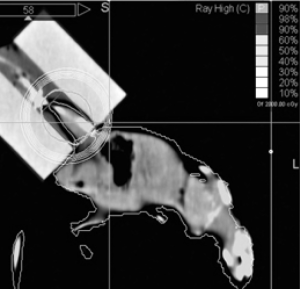
The CT volume dataset served for target definition and delineation of the mouse’s body as organ at risk (OAR). The prescription dose was 18 Gy, as earlier proposed by Boldrin et al. (3). Because of the system’s inherent inhomogeneity of dose distribution, a maximum of 19, 21 Gy was accepted in the targeted leg with the 90% isodose line completely enclosing the target region (Figure 2).
Treatment procedure
All 16 mice were treated with a single irradiation fraction of 18 Gy utilizing an image guided robotic system equipped with fiducial tracking software specialized for radiosurgical irradiation procedures (CyberKnifeR Radiosurgery System, Accuray Inc. Sunnyvale CA). The image-guided tracking system uses stereoscopic radiographs of the target, which are compared with previously generated digitally reconstructed radiographs (DRR) calculated from the planning CT dataset. Attached fiducial markers are used for superpositioning. The live images are attuned with the DRRs and the three-dimensional correction values are employed by the robotic treatment couch.
The mice were placed in the customized acrylic mold with attached fiducial markers (see Figure 1) which were tracked by the kilo volt (kV) image guiding system.
The irradiated leg was fixed with an elastic rubber band with light tension to keep it in extended position.
After the five minute treatment procedure the animals were put back in their transportation box and left alone with water supply at libitum.
Results
Total of 16 mice could be irradiated as prevised by protocol without any signs of acute irradiation toxicity or side effects caused by anesthesia.
All mice survived the time period until planned scarification after 8, 21 and 49 days, respectively.
The procedure was straight forward and the irradiation process itself took 5 minutes per animal to apply the total dose of 18 Gy. No injuries have been caused by the procedure or the fixation technique.
All animals have been able to move and run around in their box after irradiation as they did before the procedure. No acute toxicity has been noticed.
Histologic outcome
The histological work-up of muscle tissue which has not been irradiated typically shows signs of regeneration after intramuscular injection of cardiotoxin (Figure 3A). In contrary to this finding the irradiated muscle shows extensive degeneration and muscle destruction without any signs of regeneration if cardiotoxin was injected (Figure 3B). Hence irradiation seems to inhibit muscle regeneration without destroying the muscle itself.
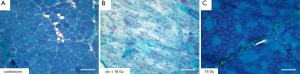
This could be proved examining the irradiated muscle, which revealed a typical and normal structure, demonstrating that the irradiation itself did not lead to necrosis or destruction of the muscle by the applied radiation dose (Figure 3C).
Discussion
Endorsed by a translational research project to investigate repair mechanisms of muscle tissue a model of targeted muscle irradiation to inactivate intrinsic muscle regeneration had to be established. Since irradiation side effects to the immune deficient mice should be minimized a precise and focused irradiation technique was necessary. We developed a treatment algorithm to apply image guided robotic radiosurgery in order to give a highly focused irradiation dose to the right mouse hind leg.
Tasks like dose planning, mouse positioning and targeting as well as fast and reproducible dose application had to be solved. A literature search found few publications of a dedicated small animal irradiation system utilizing a kV beam energy source (5-7). Three publications describe prototype irradiation systems (8-10). However, there are no publications dealing with the positioning and tracking with robotic image guided precision irradiation of limited body parts of mice using a commercially available robotic linear accelerator. Regarding the LINAC system we used, only one publication describes the precision irradiation of rat brains utilizing a customized frame and fixation device (11).
The required dose to suppress muscle regeneration was set to 18 Gy in our protocol, as suggested by Boldrin et al. for muscle irradiation (3).
Conclusions
Our herein described procedure offers a safe and easy to conduct procedure to irradiate selected areas of small animals like mouse extremities. The method is reproducible and could be repeated for several treatment series.
The survival of all animals in accordance with the protocol as well as the consistent histological findings regarding the irradiated muscle tissue confirms the expected outcome of the procedure (12).
This irradiation procedure is planned to be applied in future investigations since it is a feasible and reproducible method, which can be easily applied, especially in small animal models.
Single-session robotic radiosurgery with 18 Gy can inhibit muscle regeneration effectively without destroying the muscle or causing any measurable side effects.
The application of cardiotoxin in combination with an irradiation dose of 18 Gy to the anterior tibial muscle of immunodeficient mice inhibits the capacity of regeneration and therefore offers a robust model to evaluate the effect of implanted muscle fibers with stem-cells.
Acknowledgments
The authors thank Gabriele Thorvesten and Birgit Scheibner for their support during the irradiation procedures.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mamchaoui K, Trollet C, Bigot A, et al. Immortalized pathological human myoblasts: towards a universal tool for the study of neuromuscular disorders. Skelet Muscle 2011;1:34. [Crossref] [PubMed]
- Quinlan JG, Cambier D, Lyden S, et al. Regeneration-blocked mdx muscle: in vivo model for testing treatments. Muscle Nerve 1997;20:1016-23. [Crossref] [PubMed]
- Boldrin L, Neal A, Zammit PS, et al. Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated. Stem Cells 2012;30:1971-84. [Crossref] [PubMed]
- Verhaegen F, Granton P, Tryggestad E. Small animal radiotherapy research platforms. Phys Med Biol 2011;56:R55-83. [Crossref] [PubMed]
- Matinfar M, Iordachita I, Ford E, et al. Precision radiotherapy for small animal research. Med Image Comput Comput Assist Interv 2008;11:619-26. [PubMed]
- Matinfar M, Gray O, Iordachita I, et al. Small animal radiation research platform: imaging, mechanics, control and calibration. Med Image Comput Comput Assist Interv 2007;10:926-34. [PubMed]
- Wong J, Armour E, Kazanzides P, et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys 2008;71:1591-9. [Crossref] [PubMed]
- Stojadinovic S, Low DA, Hope AJ, et al. MicroRT-small animal conformal irradiator. Med Phys 2007;34:4706-16. [Crossref] [PubMed]
- Lindsay P, Ansell S, Moseley D, et al. Development of An Image-Guided Conformal Small Animal Irradiation Platform. Medical Physics 2008;35:2695. [Crossref]
- Zhou H, Rodriguez M, van den Haak F, et al. Development of a micro-computed tomography-based image-guided conformal radiotherapy system for small animals. Int J Radiat Oncol Biol Phys 2010;78:297-305. [Crossref] [PubMed]
- Psarros TG, Mickey B, Gall K, et al. Image-guided robotic radiosurgery in a rat glioma model. Minim Invasive Neurosurg 2004;47:266-72. [Crossref] [PubMed]
- Marg A, Escobar H, Gloy S, et al. Human satellite cells have regenerative capacity and are genetically manipulable. J Clin Invest 2014;124:4257-65. [Crossref] [PubMed]


