Early outcomes of exon 11 mutants in GIST treated with standard dose Imatinib
Introduction
The introduction of imatinib mesylate (IM) for the treatment of gastrointestinal stromal tumors (GIST) has altered the natural history and treatment paradigms of this previously near fatal disease (1-4). While the identification of immunoreactivity to CD117 (KIT receptor tyrosine kinase) and DOG1 (Discovered on GIST1) have been established as prerequisites for a pathological diagnosis of GIST (5), it is the mutations in the KIT genes or platelet derived growth factor receptor-alpha (PDGFRA) genes that have emerged as the translational link between diagnosis, prognosis and prediction of outcomes for patients with GIST. Approximately 85% of adult patients with GIST possess gain of function mutations in the KIT (75%) and PDGFRA (10%) genes and these mutations act by activation of KIT signalling pathways (5-7).
It is well recognized that the exon 11 and exon 9 activating KIT mutations have differential responses and outcomes with IM in advanced/metastatic GIST, with current recommendations allowing a dose of IM 800 mg daily for exon 9 KIT mutants as opposed to the standard 400 mg daily dose for exon 11 mutants (8-11). On the other hand, recommendations for the adjuvant and neoadjuvant treatment of operated GIST rely on the various classifications predicting recurrence, based on size, site and mitotic index, without taking into account tumor genotype (6,12-14).
Increasing research has revealed that the exon 11 mutant subset itself is a heterogeneous cohort in terms of biological behaviour. Operated patients with mutation in codon 557-558 of exon 11 have lower relapse free survival (RFS) rates compared to alterations in other codons, with a majority of the data emerging from studies in patients not treated with IM (15,16). However long term results from the BFR14 trial, in advanced inoperable GIST, have confirmed that the codon 557-558 mutation cohort has greater sensitivity to IM, but also develops secondary resistance rapidly compared to other cohorts (17). Whether similar findings are observed in the operated setting remains to be seen.
With this background, we conducted a retrospective exploratory study to evaluate early outcomes in a purely exon 11 mutated cohort across metastatic and operable subsets, treated with IM. Besides survival of the entire cohort, we also examined how the codon 557-558 mutation cohort compared against other exon 11 KIT mutants.
Materials and methods
A prospective GIST database has been maintained at Tata Memorial Centre (TMC) from May 2008 onwards. Patients had to satisfy the following criteria for entry into study:
- Confirmed histological diagnosis of GIST with adequate tissue for KIT analysis;
- Exon 11 KIT mutant.
Data for baseline demographics, tumour characteristics, details of treatment with IM (as neoadjuvant, adjuvant or in advanced disease), surgery and surgical outcomes, and outcomes with relation to progression, recurrence or death was accessed from electronic medical records and telephonic follow-up. Patients were divided into localized operable, locally advanced or metastatic GIST based on initial presentation. Our institution criteria for locally advanced GIST has been published previously (13). Operable patients (localized and locally advanced) were classified for recurrence risk as per the AFIP criteria (6).
The study has obtained ethics approval: Ethics committee approval: 1015/1519/002 (IEC TMH, Parel, Mumbai).
Testing for KIT mutations and groups
Archived formalin-fixed paraffin-embedded tissues (FFPE) of histologically and immunohistochemically proven GIST tumour samples were used for testing KIT exons 9, 11, 13 and 17 by PCR. Purified PCR products were subjected to direct DNA sequencing in both directions using BigDye v3.1 cycle sequencing kit (Applied Biosystems, USA). Sequences were analysed using sequence analysis softwares SeqScape® (Applied Biosystems) and Chromas Lite and were compared with the wild-type KIT reference sequence, with the mutations being reported as per the recommendations of the Human Genome Variation Society (HGVS). The reference sequence used in this study is KIT (Gene ID 3815). The dbSNP, COSMIC and Ensemble were referred before considering the abnormal results as ‘novel mutations’. Samples which were non-amplified, with noise or with non-readable sequences, were repeated once before considering them as uninterpretable.
Complex mutations: complex mutations in which inframe deletions were associated with second mutation within exon 11 and these second mutations were point mutations, insertions and duplications.
Inframe deletions: codons 557 and 558 (6 bp deletions) in KIT exon 11 are the deletion hot spots in GISTs affecting the regulatory juxtamembrane domain of Kit gene resulting in gain of function mutations (18). Inframe deletions more than 6bp was considered as large deletions and mutations less than 6bp were considered as small deletions.
Exon 11 mutations were grouped and analysed separately as mutually non-exclusive groups:
Method 1: the first grouping divided cohorts into 3 sub-groups (based on the division used by the BFR14 trial group):
- Alterations in codons upstream to 557, i.e., codon 556 and prior (G1);
- Alterations in codons 557-558 (G2);
- Alterations in codons downstream to 558, i.e., codon 559 and downstream (G3).
Method 2: the second group divided cohorts into 2 subsets:
- Large deletions, involving ≥6 base pairs (D1);
- Mutations not involving large deletions (D2).
The reason for dividing cohorts as above is because large deletions encompassing codons 557-558 would be included in the G1 cohort if the initial codon was identified upstream, thereby by preventing us from examining whether large deletions individually behave differently in terms of biology.
Statistical considerations
Event free survival (EFS) was calculated in patients with advanced GIST from the date of diagnosis to the date of progression, death or loss to follow up. EFS was calculated in patients with localized and locally advanced GIST treated with curative intent (including neoadjuvant IM, Radical surgery, and adjuvant IM) from the date of diagnosis to the date of recurrence, death, or loss to follow up.
Descriptive statistics were used to express baseline demographics, patient and tumour characteristics, and treatment modalities administered. Median EFS for the various exon 11 subgroups was estimated by the Kaplan-Meier method and compared using the log-rank test. Log rank test was used to identify univariate prognostic factors for EFS. Median overall survival was not assessed due to short follow up. All variables were evaluated for multivariate analysis by the Cox regression analysis, irrespective of results of univariate analysis. Statistical analysis was performed using SPSS software, version 20.
Results
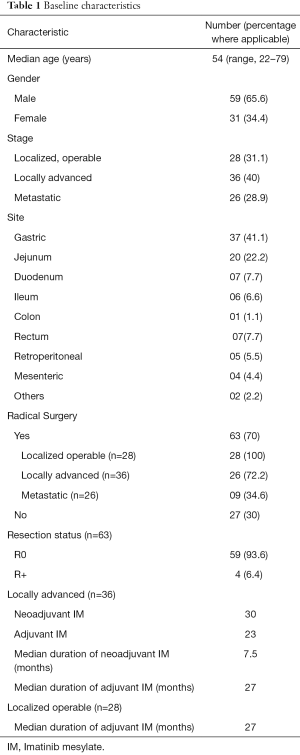
Baseline characteristics (Table 1)
Full table
The median age of the entire population was 54 (range, 22–79) years. A total of 26 patients (28.9%) were metastatic at presentation, while 28 (31.1%) and 36 (40%), were localized operable and locally advanced, respectively. The commonest site of primary was gastric, in 37 patients (41.1%), while other common sites included jejunum in 20 (22.2%), duodenum and rectum in 7 patients (7.7%), respectively.
Of the patients who underwent upfront surgery (n=28), 25 patients (89.2%), were high risk, 1 patient (3.5%) was low risk and 2 patients (7%) could not be classified by AFIP classification. Thirty four patients (n=36; 94.4%) of the locally advanced patients were high risk, while 2 patients (5.4%) could not be classified due to the location of the primary tumor (Mesenteric and gallbladder, respectively) (Supplementary Table S1).

Full table
A total of 63 patients (70%) underwent radical intent surgery. This included 28 patients (n=28; 100%) with localized operable GIST, 26 patients (n=36; 72.2%) with locally advanced disease and 9 patients (n=34; 34.6%) with metastatic disease (multivisceral resections). Of the surgical cohort, 59 patients underwent an R0 resection (n=63; 93.6%), while the remaining 4 patients (n=63; 6.4%) underwent an R+ resection. Three of the patients undergoing R+ resection had metastatic disease, while one patient with locally advanced disease had an R+ resection.
Of the locally advanced cohort (n=36), 30 received neoadjuvant IM, for a median duration of 7.5 months. Post-surgery (n=26), 24 received adjuvant IM for a median duration of 27 months.
Twenty eight patients were localized operable upfront. Of these patients, 22 patients received adjuvant IM for a median duration of 27 months.
Further details regarding treatment are mentioned in Supplementary Table S1.
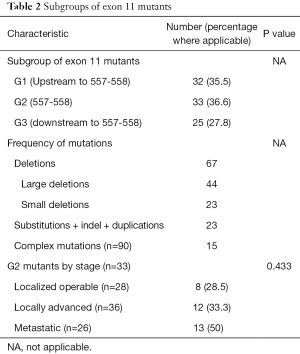
Exon 11 mutant status (Table 2)
Full table
The frequency of the different mutations, classified as previously explained, seen in the exon 11 mutants is described in Table 2. Briefly, deletions were seen in 67 patients, of which 44 (n=90; 48.8%) were large deletions and 23 (n=90; 25.5%) were small deletions. Complex mutations were seen in 15 (n=90; 16.7%) patients.
G1 mutations were seen in 32 patients (35.5%), G2 in 33 patients (36.6%) and G3 in 25 patients (27.8%) respectively. There was no significant differences in the proportion of patients of G2 cohort between the localized, locally advanced and metastatic subgroups (P=0.433).
EFS and survival analysis (Table 3)
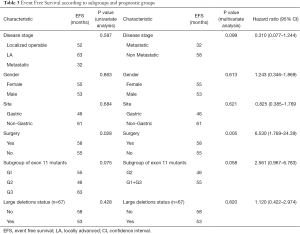
Full table
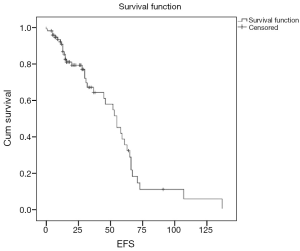
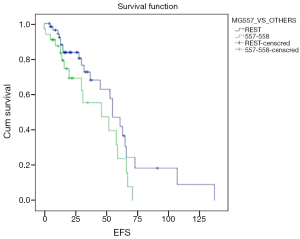
With a median follow of 26 months, 40 patients had an event (n=90; 44.4%) as specified. Median estimated EFS for the entire cohort was 55 months (Figure 1). On univariate analysis, patients undergoing surgery had a longer EFS than those who did not undergo surgery (58 vs. 55 months; P=0.028). On multivariate analysis, patients undergoing surgery continued to have a longer EFS (P=0.005; HR –6.53; 95% CI: 1.769–24.39). There was a trend on univariate and multivariate analysis for difference in EFS (Figure 2) between the G2 versus (G1+G3) cohort, but this did not reach statistical significance (univariate analysis: P=0.075; multivariate analysis: P=0.058). Non-metastatic patients tended to do better than metastatic patients and this approached, but did not reach statistical significance on multivariate analysis (univariate analysis: P=0.587; multivariate analysis: P=0.099). Other factors selected for evaluation as prognostic factors did not reach statistical significance.
Discussion
The heterogeneity in the tumor biology of GIST as well as differential response to tyrosine kinase inhibitors is an area of ongoing research. The knowledge of KIT exon 11 mutants having a longer PFS on standard IM and KIT exon 9 mutants potentially benefitting from 800 mg doses of IM has emerged in the advanced setting (19). The Contica GIST study (15), clearly showed that gastric GIST with a KIT del 557-558 had an inferior disease free survival compared to other mutants. A larger study, comprising 11 population based series (n=3,067; mutation analysis-1505), but untreated with IM, concluded that patients with PDGFRA mutations and those with KIT exon 11 duplication/single codon deletion mutations have a favorable recurrence free survival (RFS) with surgery alone (20). Conversely, in the adjuvant setting in operated tumors, mutation status has not replaced standard criteria with regard to the need for adjuvant IM, although data from long term follow up of the ACOSOG Z9001 Trial has suggested that KIT exon 11 deletions had maximal benefit from 1 year of adjuvant IM compared to other molecular subsets (21).
However, a majority of studies, with the notable exception of the BFR-14 study (17), have focussed on the outcomes of these cohorts without further subdividing the KIT exon 11 mutants and examining the potentially different responses of these mutants to IM. There also remains the question of how to handle neoadjuvant IM in patients with locally advanced GIST with respect to mutation status, given the sparse data (13).
With this background, our study attempts to identify early predictive signals in the treatment of the KIT exon 11 mutant cohort and whether the biological correlates shown in the non-IM treated cohort as well as IM treated advanced GIST cohort are seen in our patient population.
The estimated median EFS seen our study was 55 months, with the metastatic cohort having an estimated median survival of 32 months. This is markedly better than the 18–24 months seen with advanced GIST as a whole cohort in various studies, but in line with what has been seen in the BRF 14 study (PFS: 39.4 months) (17,22). The operable cancers (including locally advanced) had a median EFS of 58 months (5 year EFS: 50%), which is lower than published data (14,21) for operated and locally advanced cancers. The differences can be attributed to the small sample size, short follow-up and real world nature of the data compared to trial data.
Despite the above mentioned factors, there appears to be a trend, as in the advanced setting, towards the mutation 557-558 cohort having a shorter EFS on IM (univariate analysis, P=0.075; multivariate analysis, P=0.058). The shorter PFS/EFS associated with mutation 557-558 groups has been explained by the Trp557 being identified as having inhibitory roles in the control of receptor kinase activity as well substitution of proline for Lys558 leading to a high degree of constitutive receptor phosphorylation (23). While IM only has inhibitory effect in a non-phosphorylated KIT receptor and thus resistance in the mutation 557-558 can be explained, whether non-KIT related mechanisms of resistance to IM also have a role to play needs further evaluation (24). In vitro studies evaluating the IC50 of IM in the mutation 557-558 cohort are required to identify the appropriate doses and benefit of IM in this cohort. Such studies further become relevant, considering the routine use of neoadjuvant IM in locally advanced GIST as well as adjuvant IM for a duration of 3 years in intermediate/high risk GIST. Additionally, a longer follow up may give us further insight into the behaviour of this cohort, as Martin Broto et al., have suggested that the mutation 557-558 in exon 11 may exercise an adverse prognostic effect only in the first 4-year time period and not later (25).
The importance of surgery in operable GIST was renewed, with the surgical group showing superior outcomes on univariate and multivariate analysis (univariate, P=0.028; multivariate, P=0.005). While the numbers in this study are small, the similar survivals of the upfront operable GIST and locally advanced GIST, who were treated with majorly treated with neoadjuvant IM, is suggestive of the feasibility of neoadjuvant IM and its benefits. It also suggests that certain adverse biological characteristics in KIT exon 11 subgroups may be obviated by surgery.
Variables such as age, gender, tumor location, and large deletions did not achieve statistical significance as prognostic factors in our analysis. While there is some correlation between gastric location, larger size and the presence of mutation 557-558 showing a lower RFS, the predominantly high risk nature of our cohort meant that a majority of operable/operated patients were candidates for adjuvant IM, thereby shifting the onus of this analysis to evaluation of performance of the exon 11 cohort on IM. While Contica GIST study (15) showed poorer outcome of gastric GIST patients with mutation 557-558, our study showed trend towards poorer outcome in that group irrespective of location.
Our analysis is a useful addition to the growing literature regarding the heterogeneous nature of the exon 11 mutant KIT GIST and this might prompt the need to plan treatment strategies based on individual mutant cohorts. The short duration of follow up is an important caveat in this study. Again, the predominantly high risk nature of our cohort does not answer questions regarding the intermediate risk subgroup of GIST with exon 11 mutants. We have also not analysed outcomes based on the individual components of the AFIP criteria as a majority of the patients in this study were high risk.
In conclusion, our study of a pure exon- 11 mutant cohort shows a signal towards the mutation 557-558 mutant maintaining its aggressive biological behaviour even in patients receiving IM across the metastatic and operated setting. Long term follow up is required in a potentially larger population to further validate these findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by local ethics committee: 1015/1519/002 (IEC TMH, Parel, Mumbai), and written informed consent was obtained from all patients.
References
- von Mehren M, Heinrich MC, Joensuu H, et al. Follow-up results after 9 years (yrs) of the ongoing, phase II B2222 trial of imatinib mesylate (IM) in patients (pts) with metastatic or unresectable KIT+ gastrointestinal stromal tumors (GIST). ASCO Meet Abstr 2011;29:10016.
- Bauer S, Rutkowski P, Hohenberger P, et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib -- analysis of prognostic factors (EORTC-STBSG collaborative study). Eur J Surg Oncol 2014;40:412-9. [Crossref] [PubMed]
- DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg 2013;258:422-9. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Corless CL. Gastrointestinal stromal tumors: what do we know now? Mod Pathol 2014;27 Suppl 1:S1-16. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [PubMed]
- Tarn C, Godwin AK. The molecular pathogenesis of gastrointestinal stromal tumors. Clin Colorectal Cancer 2006;6 Suppl 1:S7-17. [Crossref] [PubMed]
- Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [Crossref] [PubMed]
- Debiec-Rychter M, Dumez H, Judson I, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2004;40:689-95. [Crossref] [PubMed]
- Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 2006;42:1093-103. [Crossref] [PubMed]
- ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii21-6. [Crossref] [PubMed]
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005;29:52-68. [Crossref] [PubMed]
- Ramaswamy A, Ostwal V, Shetty O, et al. Neoadjuvant Imatinib in Locally Advanced Gastrointestinal stromal Tumours, Will Kit Mutation Analysis Be a Pathfinder? J Gastrointest Cancer 2016;47:381-8. [Crossref] [PubMed]
- Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013;20:2937-43. [Crossref] [PubMed]
- Wozniak A, Rutkowski P, Schöffski P, et al. Tumor genotype is an independent prognostic factor in primary gastrointestinal stromal tumors of gastric origin: a european multicenter analysis based on ConticaGIST. Clin Cancer Res 2014;20:6105-16. [Crossref] [PubMed]
- Martín J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol 2005;23:6190-8. [Crossref] [PubMed]
- Patrikidou A, Domont J, Chabaud S, et al. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer 2016;52:173-80. [Crossref] [PubMed]
- den Dunnen JT, Dalgleish R, Maglott DR, et al. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum Mutat 2016;37:564-9. [Crossref] [PubMed]
- Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53. [Crossref] [PubMed]
- Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol 2015;33:634-42. [Crossref] [PubMed]
- Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014;32:1563-70. [Crossref] [PubMed]
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3-14. [Crossref] [PubMed]
- Ma Y, Cunningham ME, Wang X, et al. Inhibition of spontaneous receptor phosphorylation by residues in a putative alpha-helix in the KIT intracellular juxtamembrane region. J Biol Chem 1999;274:13399-402. [Crossref] [PubMed]
- Szucs Z, Thway K, Fisher C, et al. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol 2016. [cited 2016 Nov 10]; Available online: http://www.futuremedicine.com/doi/full/10.2217/fon-2016-0192
- Martin-Broto J, Gutierrez A, Garcia-del-Muro X, et al. Prognostic time dependence of deletions affecting codons 557 and/or 558 of KIT gene for relapse-free survival (RFS) in localized GIST: a Spanish Group for Sarcoma Research (GEIS) Study. Ann Oncol 2010;21:1552-7. [Crossref] [PubMed]