The KEY to the end of chemotherapy in non-small cell lung cancer?
Until recently, advances in the treatment of non-small cell lung cancer (NSCLC) has been with the use of molecular targeted therapy in tumors harboring oncogenic drivers such as epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) or ROS1 gene rearrangement (1-3). However, a majority of non-Asian NSCLC do not harbor an actionable driver oncogene (4) and a platinum doublet with or without bevacizumab is still the standard of care in the first line setting (5).
The introduction of PD-1 and PD-L1 immune checkpoint inhibitors has altered the therapeutic landscape in advanced NSCLC. In the second line setting, phase III trials have demonstrated the superiority of immune checkpoint inhibitors over docetaxel. CHECKMATE 017 and CHECKMATE 057 were phase III studies of nivolumab versus docetaxel in patients who have progressed on a platinum-based chemotherapy. CHECKMATE 017 recruited patients with squamous NSCLC whilst CHECKMATE 057 enrolled non-squamous NSCLC. In CHECKMATE 017, the overall survival (OS) was 9.2 vs. 6 months, (HR =0.59, P<0.001) (6) and in CHECKMATE 057, the OS was 12.2 vs. 9.4 months (HR =0.73, P=0.002) (7). Toxicity profile favored the nivolumab arm. In a phase II/III study (KEYNOTE 010), patients with pre-treated PDL1 +ve (defined as tumour proportion score (TPS) of at least 1%) advanced NSCLC were randomized to pembrolizumab 2 or 10 mg/kg every 3 weeks or docetaxel. In the overall population, the OS in patients treated with pembrolizumab 2 and 10 mg/kg was 10.4 (HR =0.71, P=0.00076) and 12.7 months (HR =0.61, P=0.0001), respectively compared with docetaxel with an OS of 8.5 months (8). Of note, in patients with a TPS ≥50%, the OS with pembrolizumab 2 and 10 mg/kg was 14.9 months (HR =0.54, P=0.0002) and 17.3 months (HR =0.50, P<0.0001 months), respectively versus 8.2 months with docetaxel. Recently, in a phase III study of atezolizumab versus docetaxel in pre-treated advanced NSCLC unselected for PDL-L1 expression (OAK study), the OS was 13.8 vs. 9.6 months (HR =0.73, P=0.0003). Atezolizumab was beneficial regardless of PD-L1 expression and histology (9). This led to the FDA approval of atezolizumab in patients with pre-treated advanced NSCLC.
Given the activity of immune checkpoint inhibitors in pre-treated patients, studies have examined the role in the 1st line setting. Preliminary activity of pembrolizumab in the 1st line setting was suggested in a phase I study (KEYNOTE 001) (10). In particular, pembrolizumab was highly active in patients with high PD-L1 expression. In patients with a TPS of ≥50%, 1–49% and <1%, the objective response rate (ORR) was 58.3%, 17.4%, and 10%, respectively, the progression free survival (PFS) was 12.5, 4.2, and 3.5 months, respectively and the OS was not reached, 19.5, and 14.7 months, respectively (11). Results from KEYNOTE 001 and KEYNOTE 010 supported PD-L1 expression as a predictive biomarker for pembrolizumab.
These promising results have led to the highly anticipated results of KEYNOTE 024, reported by Reck et al. (12). KEYNOTE 024 was a randomized phase III study that compared pembrolizumab 200 mg every 3 weeks for 2 years versus platinum doublet chemotherapy in 305 treatment-naïve advanced NSCLC patients. Key eligibility criteria included advanced NSCLC, high tumor PD-L1 expression (defined as TPS of ≥50%), and ECOG 0–1. Patients with sensitising EGFR mutations or ALK rearrangement, or untreated brain metastases were excluded. The primary end point was PFS, secondary end points were OS, ORR and safety. The trial was stopped after second interim analysis on the recommendation of the data and safety monitoring committee. An improvement in PFS was seen with pembrolizumab compared with compared to chemotherapy (10.3 vs. 6 months, HR=0.5; 95% CI: 0.37 to 0.68; P<0.001). The benefit was seen in all subgroups. An improved OS with pembrolizumab was also seen (estimated 6months OS 80.2% vs. 72.4%, HR=0.6, P=0.005). The safety profile of pembrolizumab was consistent with that seen in previous studies, and lower than that with chemotherapy (grade 3/4 adverse events 27% vs. 53%). In addition, pembrolizumab was associated with an improvement in quality of life and a longer time to deterioration for cough, dyspnea and chest pain (13). Results from the chemotherapy arm were consistent with previous first line studies, with ORR of 30% and PFS of 6 months.
Several findings from this study should be highlighted. Firstly, a high ORR of 44.8%, impressive for single agent therapy and similar to ORR of 58% reported in KEYNOTE 001 in the TPS ≥50% cohort. Secondly, no delay in responses was seen with pembrolizumab with a median time to response of 2.2 months, the same as that for chemotherapy. Another important feature was, despite a high cross over rate (43%) from the chemotherapy arm to pembrolizumab, OS benefit was still maintained. Finally, the optimal dose of pembrolizumab remains unclear. In KEYNOTE 024, pembrolizumab was administered at a fixed dose of 200 mg every 3 weeks whereas in KEYNOTE 010, pembrolizumab was dosed at 2 and 10 mg/kg (8). A pharmacokinetic study (14) reported pembrolizumab 200 mg provided similar exposure distribution as weight-based dosing regimen of 2 mg/kg. Given the high costs of immunotherapy, future studies are required to establish a minimum effective dose of pembrolizumab as these can potentially reduce costs in patients with low body weight.
A similarly designed phase III study of nivolumab in treatment naïve advanced NSCLC (CHECKMATE 026) was presented recently. In this study, 541 patients with tumor PD-L1 expression ≥1% were randomized to nivolumab or a platinum doublet. In patients with tumor PD-L1 ≥5%, the PFS was 4.2 vs. 5.9 months (HR =1.15, P=0.25), OS was 14.4 vs. 13.3 months (HR =1.02) and ORR was 26.1% vs. 33.5%. The reasons for the difference in results between KEYNOTE 024 and CHECKMATE 026 are unclear but may be due to differences in patient selection. In CHECKMATE 026, an unusually high number of patients received palliative radiotherapy prior to starting treatment. The biomarker assays were different with the 28-8 assay not validated prospectively and patients were selected based on a tumor PD-L1 expression cut-off of 1% (Table 1).

Full table
Despite the improvement in outcomes seen in KEYNOTE 024, several important issues remain. Firstly, only about 30% of patients have high tumor PD-L1 expression, and for the remaining 70% of patients with low or absent PD-L1 expression, chemotherapy is still the current standard. Improving treatment for this large group of patients remains crucial. This will be addressed by the ongoing KEYNOTE 042 and other studies (Table 2). Secondly, despite the improvement in PFS, progression still occurs (median 10.3 months). Thirdly, patients with EGFR mutations or ALK translocation were excluded. To address these issues, trials of checkpoint inhibitor in combination with chemotherapy, or targeted therapy or another checkpoint inhibitor are ongoing (Table 2).
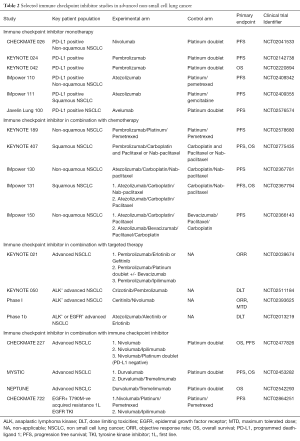
Full table
Early phase studies of checkpoint inhibitors with chemotherapy in the first line setting have been reported. In a phase I study of nivolumab and platinum based doublet, safety was as expected but treatment discontinuation due to adverse events was 21%. The ORR was 33–47% and the 2-year OS was 25–62% (15). In a randomized phase II study (KEYNOTE 021) comparing pembrolizumab and platinum doublet versus platinum doublet in PD-L1 unselected non-squamous NSCLC, results were highly encouraging with a PFS of 13 vs. 8.9 months and an ORR of 55% vs. 29% (P=0.0016), respectively. In patients with TPS ≥50%, the ORR was 80% for the combination arm. Toxicities were manageable and did not lead to higher discontinuation rates (11% vs. 13%) (16). Phase III studies of combination immune checkpoint inhibitors and chemotherapy versus chemotherapy are ongoing in both squamous and non-squamous NSCLC (Table 2).
Several early phase studies of immune checkpoint inhibitors and molecular targeted therapy in the first line setting are ongoing (Table 2). Preliminary results of first line durvalumab and osimertinib in EGFR mutant NSCLC reported an ORR of 70% but grade 3/4 toxicities was unacceptably high at 59% and any grade interstitial lung disease was 64% (17). High rates of grade 3/4 treatment related toxicity (55%) was also reported in a phase I study of durvalumab and gefitinib (18). The combination of erlotinib with atezolizumab reported an ORR of 75% and disease control rate of 95%, with 39% grade 3/4 adverse events (19).
A phase I study of ipilimumab and nivolumab as first line treatment of advanced NSCLC has recently been reported (20). Treatment discontinuation due to treatment related toxicity was 11% and the ORR was 38–47% overall and 57% in PD-L1 +ve (≥1%). Notably, an ORR was 92% in patients with high tumor PD-L1 expression (≥50%). Whilst this result should be viewed with caution given the small sample size and possible selection bias in a phase I study, it suggests the improved efficacy of combination immune checkpoint inhibitors in tumors with high PD-L1 expression.
In conclusion, KEYNOTE-024 is a landmark trial that has established the role of first line pembrolizumab in patients with PD-L1 positive, advanced NSCLC without an oncogenic alteration. Patients with high tumor PD-L1 expression were selected using a prospectively validated PD-L1 assay. Studies addressing the efficacy of combination therapies in tumors without high PD-L1 expression are ongoing.
Acknowledgments
RAS is supported by the National Medical Research Council NMRC/CG/012/2013, the National Research Foundation Singapore, the Singapore Ministry of Education under its Research Centres of Excellence initiative.
Footnote
Conflicts of Interest: RAS has received honorarium from AstraZeneca, BMS, Boehringer Ingelheim, Lilly, Merck, Novartis, Pfizer, Roche, Taiho; and research funding from AstraZeneca. YH has no conflict of interest of interest to declare.
References
- Mok TS, Wu Y, Thongprasert S, et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non–small cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-v27. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous cell non–small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3,open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Hui R, Gandhi L, Costa EC, et al. Long-term OS for patients with advanced NSCLC enrolled in the KEYNOTE-001 study of pembrolizumab (pembro). J Clin Oncol 2016;34:abstr 9026.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1 positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. Health-related quality of life for pembrolizumab vs. chemotherapy in advanced NSCLC with PD-L1 TPS ≥50%: Data from KEYNOTE-024. Presented at the International Association for the Study of Lung Cancer 17th World Conference on Lung Cancer (WCLC) 2016. Abstract #PL04a.01.
- Freshwater T, Stone J, de Greef R, et al. Assessment of Pembrolizumab (MK-3475) Dosing Strategy Based on Population Pharmacokinetics and Exposure-Response Models. Journal of Pharmacokinetics and Pharmacodynamics 2015;42:S15.
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol 2016;34:2969-79. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Ahn M, Yang JC, Yu H, et al. Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 2016;11:S57-S166. [Crossref] [PubMed]
- Gibbons DL, Chow LQ, Kim DW, et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): A phase I expansion in TKI-naïve patients (pts) with EGFR mutant NSCLC. J Thorac Oncol 2016;11:S79. [Crossref] [PubMed]
- Ma BB, Rudin CM, Cervantes A, et al. 441O Preliminary safety and clinical activity of erlotinib plus atezolizumab from a Phase Ib study in advanced NSCLC. Ann Oncol 2016;27:mdw594.005.
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]


