Opposite effects of Agrimonia pilosa Ledeb aqueous extracts on blood coagulation function
Introduction
The hemostatic system has several important functions preventing individuals from thrombosis or bleeding, and normal physiology constitutes a delicate balance between procoagulant and anticoagulant properties of this system, and a deficiency or exaggeration of any one may lead to either hemorrhage or vascular thrombosis, respectively (1). Hemostasis requires both platelets and the coagulation system, and platelet, coagulating factors and blood flow are all contributed to hemostasis and thrombosis (2). However, activation of platelet and coagulation factors, and abnormal flow velocity or vascular wall, tissue factor expression, interaction of platelet with other factors may play important roles in abnormal blood coagulation and the process of thrombosis (3). Normal blood clotting and thrombosis are thought to occur by the same pathway (4). On the other hand, besides abnormal platelet function, the deficiencies of extrinsic, intrinsic or common pathways are the important causes of hemorrhagic disorders (4).
For the prevention and treatments for bleeding or thrombosis, lots of drugs have been used as hemostatic or antithrombotic agents. Recently, plant derived natural products possessing huge ethnopharmacological have given priority to treatments for thrombosis and bleeding (5,6). In China and other Asian countries, Agrimonia pilosaLedeb (APL) has been used traditionally for treatments of abdominal pain, sore throat, headaches, bloody discharge, parasitic infections and tumor since several centuries (7). Moreover, for thousands of years, APL also has been widely used as a hemostatic agent in bleeding patients with traumatic injury in China (8), which reveals that APL can promote blood clotting, and also suggests that it has effect on blood coagulation function. On the other hand, some studies have found that APL has in vitro antiplatelet, anticoagulation and antithrombosis effects (9,10). These studies seem to demonstrate contradictory results about the effects of APL on blood coagulation.
As the easily gained and important extracts, APL aqueous extracts are rich of some active ingredients including ionized calcium, phosphate, trace elements, flavonoid compounds and phenols (9,11,12). Therefore, much of the active ingredients may be potential materials influencing coagulating process, and there may be important significance to make their effects clear. Although many studies have provided evidences of either anticoagulant or procoagulant effects of APL in different sides, we still do not know the actual effects on coagulation function. Moreover, these studies did not fully clarify the specific mechanisms, thus the mechanisms remain unclear. In this study, we systematically examined and observed the in vitro effects of APL aqueous extracts on some tests and parameters related to coagulation function, and identified the preliminary mechanisms.
Methods
Participants
Thirty healthy volunteers including 17 males and 13 females aged from 20 to 27 years old, were recruited for this study, and all subjects had taken no medicine in two weeks before samples collecting. For experimental requires, 15 to 25 milliliters venous blood from each volunteer was collected in the morning after fasting for 8 hours or more according to routine procedures. This study was approved by local ethics committee on the use of clinical human samples for research of the hospital (ID of ethics approval: 2014KY011), and has the written informed consent of all volunteers.
Materials, reagents and chemicals
APL was collected from the medicinal garden of Zhejiang University of Technology. APL aqueous extracts (1.25 g raw herbs /mL) prepared as described (13). Mouse anti-human PAC-1-FITC, matching isotype controls (Immunotech Company, Beckman-Coulter). Adenosine diphosphate (ADP) (Sigma-Aldrich Company). Thromboplastin kit (for Prothrombin time, PT), Cephaloplastin kit (for activated partial thromboplastin time, aPTT), Test thrombin kit (for thrombin time, TT), Thrombin reagent kit (for fibrinogen, Fbg), coagulation factors (factor VII, VIII, IX, X, XI) deficient plasma, and 0.025 mol/L calcium chloride solution (Siemens Healthcare Diagnostics Products GmbH). ZLC cleaning and rinse solution (Beijing ZONCI technology, China). Other chemicals (Analytical grade, Hangzhou Huadong Pharmaceuticals, China).
Preparation of human platelet-rich and platelet-poor plasma
Human platelet-rich (PRP) and platelet-poor plasma (PPP) was prepared as described previously (14). In brief, venous blood was collected with siliconized tubes (Gongdong Medical Technology Company, Taizhou, China), and was mixed with 3.8% tri sodium citrate solution (9:1, v/v), which was used to prepare PRP by centrifugation at 200 g for 10 minutes at room temperature. Simultaneously, 6.0 IU/mL Lithium heparin was mixed with 5 mL blood sample. And PPP was obtained from the blood with the two types of anticoagulants by centrifugation at 1,500 g for 10 minutes at room temperature.
Groups and sample treatments
Referring to the study which there was significant inhibitory effect of 16 g/L APL aqueous extracts on Ecal 109 cells (15), we divided groups into 0, 4, 20, and 80 g/L according to final APL aqueous extracts concentration, respectively. And all the experiments started within one hour after sampling. PRP, PPP and whole blood were mixed with saline solution or APL extracts in cuvettes, respectively. Prior to all tests, the capped samples were incubated for 15 minutes at 37 °C in water bath (repeating 6 times independent experiments with blood samples from 6 volunteers, respectively).
Measurements of clotting time of whole blood
Clotting time (CT) of whole blood was measured in the siliconized tube referring to ACT measurements described previously (16). Six volunteers (3 males and 3 females) participated in 6 times independent experiments. In each experiment, 15 milliliters blood sample from each volunteer was collected, and 3 milliliters aliquot sample was immediately mixed with different concentrations of APL aqueous extracts in siliconized tubes, respectively. The complete blood coagulation time (in seconds) was recorded, which was CT. All the recorded time was not more than one hour.
Measurements of plasma coagulation tests, activities of coagulation factors, and calcium ion
Total 15 milliliters blood sample from each volunteer (total 4 males and 2 females), was collected to determine plasma coagulation tests and coagulation factors activities, and another six volunteers (3 males and 3 females) who were sampled the same volume of blood participated in the measurements of calcium ion in the four groups, respectively. In single test, 3 milliliters aliquot blood sample was used. The citrated PPP incubated with different concentrations of APL aqueous extracts cooled for 5 minutes at room temperature, then PT, aPTT, TT, and Fbg levels, as well as the activities of coagulation factors were immediately measured using the matched kits by automatic coagulation analyzer Sysmex CA-7000 as reported (17), And calcium ion (Ca2+) was determined by ABL-800 blood-gas analyzer (Rador, Danmark) within one hour.
Platelet aggregation tests
Four male and two female volunteers participated in 6 times of independent experiments in this part, respectively, and 15 milliliter venous blood was sampled from each one. Platelet aggregation tests were performed using aggregation analyzer (Model AggRAM, Helena Laboratories, USA) as described previously (18). In brief, 3 milliliter blood sample was used for each test, and platelet count in incubated PRP with different concentrations of APL aqueous extracts was adjusted to 200×109/L with its own PPP, 250 µL of PRP was pipetted into test tube, and 25 µL ADP (final concentration was 5 µmol/L, not pipetting ADP at rest group) was then added to induce platelet aggregation. The data was represented at the maximal aggregation percent calculated by taking ADP-induced aggregation of untreated platelet as 100%. The inhibitory percent of platelet aggregation was calculated in different concentrations of APL extracts.
Measurements of fibrinogen receptor expression
Total 225 µL PRP treated with the APL aqueous extracts and activated by ADP in platelet aggregation test was fixed with 3% paraformaldehyde (1:1, v/v) for 15 minutes at room temperature in dark place, then mixed with TEN (Tris-EDTA-NaCl) buffer solution (1:3, v/v). The treated samples were stained with equal volume of PAC-1-FITC mAbs or a matching isotype control for 10 minutes at room temperature. A flow cytometer (NAVIOS, Beckman-Coulter) was used to measure the expression of fibrinogen receptor (FIB-R) in 10, 000 cells, and the data was represented at the percent of positive cell. At the same time, the inhibitory percent was calculated.
Measurements of whole blood and plasma viscosity, and red cell aggregation index
Fifteen milliliters venous blood was sampled from each volunteer (total 3 males and 3 females), and 3 milliliters aliquot blood sample was used in each test. After the cone-plate viscometer (Beijing ZONCI technology, China) was warmed to 37 °C, heparinized whole blood with different concentrations of APL aqueous extracts was used to measure the whole blood viscosity (η1 and η200) in shear rates of 1/s and 200/s. And the plasma viscosity was measured with the PPP. According to manufacturer’s instruction, red cell aggregation index (IRCA) was calculated following the formula: IRCA=η1/η200.
Statistical analysis
Data of the results was analyzed by one way analysis of variance (ANOVA). If this analysis indicated significant differences among the group means, each group was compared using Student-Newman-Keuls (SNK) test. P value of less than 0.05 was considered statistically significant.
Results
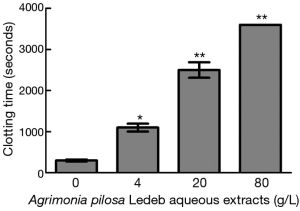
Effects of the APL aqueous extracts on clotting time
The effects of different concentrations of APL aqueous extracts on CT are presented in Figure 1. The results demonstrated that the extracts at 4 g/L significantly prolonged CT (1,100±120 seconds vs. 310±51 seconds at 0 g/L), and 80 g/L group, whole blood exhibited no coagulation after one hour. Moreover, CT increased in concentration-dependent manner (0–80 g/L of APL aqueous extracts).
Effects of the APL aqueous extracts on coagulation tests, activities of coagulation factors, and calcium ion
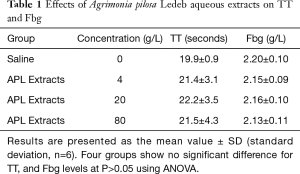
The effects of different concentrations of APL aqueous extracts on coagulation tests showed that the extracts at concentration of 4 g/L significantly shortened PT (11.3±1.1 vs. 11.9±1.2 seconds at 0 g/L), but significantly prolonged aPTT (35.5±8.8 vs. 25.5±5.1 seconds at 0 g/L), and the extracts concentration-dependently (0–80 g/L) shortened PT and prolonged aPTT. Whereas TT and Fbg levels were not significantly affected by the extracts. Detailed results are summarized in Table 1 and Figure 2. In Table 2, the results showed that the APL aqueous extracts did not significantly affect Ca2+ concentration, and the coagulation factor X activity, but concentration-dependently (0–80 g/L) increased the activity of coagulation factor VII, and decreased the activities of coagulation factor VIII, IX, and XI.
Full table

Full table
Effects of the APL aqueous extracts on platelet aggregation and platelet FIB-R expression
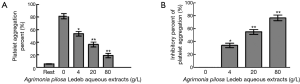
As shown in Figure 3 and Table 3, the platelet aggregation percent, and the levels of Fib-R expression in 4 g/L were significantly lower, but the inhibitory percent of the two indicators was significantly higher than that in 0 g/L, respectively. And following the extracts concentration increasing, the platelet aggregation percent and levels of Fib-R expression significantly decreased in concentration-dependent manner (0–80 g/L).
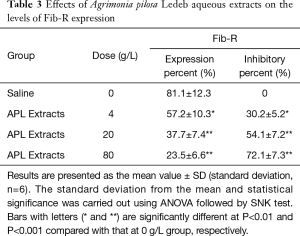
Full table
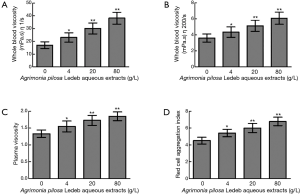
Effects of the APL aqueous extracts on blood viscosity
The whole blood viscosity in shear rates of both 1/s and 200/s, plasma viscosity, and red cell aggregation index significantly increased in 4 g/L than that in 0 g/L, and following the extracts concentration increasing, levels of all blood viscosity indicators were elevated in concentration-dependent manner (0–80 g/L). Detailed results are summarized in Figure 4.
Discussion
Clotting-time tests include PT, aPTT, TT, activated clotting time (ACT), and so on. ACT measures the clotting time of whole blood mixed with activating reagents such as Kaolin and Calcium, and is usually used to quickly monitor hemostatic function of patients undergoing heparin therapy (19,20). In the present study, we replaced ACT with the clotting time of whole blood without any activator (named as CT) to assess the effects of APL extracts. As we know, CT may reflect the in vitro total blood coagulating condition in a large part, and exhibit the interacting result of most ingredients involved in blood coagulating process excluding contacting activator, which its clinical significance might be similar to ACT. Therefore, we firstly observed the influence of APL aqueous extracts on CT to verify the general effects on coagulation function. The results demonstrated that the CT was markedly affected by 4 g/L of APL aqueous extracts, and significantly prolonged in a concentration-dependent manner. Moreover, clotting was not observed even at more than one hour treated by 80 g/L extracts. These results indicated that the extracts may inhibit blood coagulation. Therefore, APL aqueous extracts might have an anticoagulating activity macroscopically in vitro, which is probably the basic cause of the antithrombotic effect of APL in practice. To understand the causes of prolonged CT, we further investigated the effects of APL aqueous extracts on coagulation factors, platelet, and blood viscosity, which may contribute to the process of whole blood coagulating.
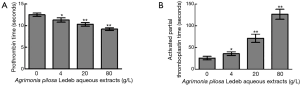
Activation of blood coagulation system can be achieved through the extrinsic or intrinsic pathway which leads activation of the common pathway and conversion of fibrinogen to fibrin for clot formation (21). As the first-line coagulation tests in hemostasis laboratory, PT and aPTT are usually used to monitor whether there are deficiencies or abnormalities of extrinsic (i.e., factor VII) and intrinsic (i.e., factor VIII, IX, and XI) coagulation pathways, respectively (1,22,23). In this section, significantly prolonged aPTT and shortened PT was observed following elevated concentrations of APL aqueous extracts, which indicated that the extracts may have an inhibitory effect on intrinsic pathway of blood coagulation, and an accelerating effect on extrinsic pathway, and the effects were also in a concentration-dependent manner. Based on the contradictory results, APL aqueous extracts seemed to demonstrate both anticoagulant and procoagulant effects on blood coagulation system, which was confusing extremely. Although there were opposite effects on either intrinsic or extrinsic pathway of coagulation, CT results indicated that APL aqueous extracts actually has an anticoagulant effect macroscopically, which was in accord with the alteration of aPTT levels. Smythe et al. have reported that there was high correlation ranging from r=0.64 to 0.67 between aPTT and ACT (24), which further supported that there would be a high correlation between CT and aPTT mentioned above. At the same time, although it was statistically significant, the effect of the extracts on PT may be trivial, and probably not indicative of an important effect totally. Therefore, we suppose that the leading effects of APL aqueous extracts are to inhibit the coagulation system, which mainly contribute to the total anticoagulant activity.
Ca2+ is a key ingredient involved in blood coagulation (25). In this study, Ca2+ was not notably influenced in the presence of APL aqueous extracts, which implied that the effects of the extracts on CT, intrinsic and extrinsic coagulation pathways may be unrelated to Ca2+, and the extracts may not be directly to interference with the action of Ca2+ in coagulation process in vitro. In blood coagulation process, coagulation factors play important roles, and they are the important materials involved in second hemostasis and blood coagulation. In this study, increased factor VII activity, and decreased activities of factor VIII, IX, and XI are in accord with shortened PT, and prolonged aPTT, respectively. Therefore, the activation of extrinsic coagulation pathway may be mainly due to the increased activity of factor VII, which is probably associated with the hemostatic effect of some ingredients in the extracts. On the contrary, inhibition of factor VIII, IX, and XI activities might not contribute to the inhibition of intrinsic coagulation pathway because the activities of the factors almost were above 50% which may not cause a prolonged aPTT. Therefore, it is likely that the APL extracts affected the color of the samples and aPTT measurements, and hence led to a prolonged aPTT. However, the results are confusing extremely. The activities of factors FX and FVII demonstrated different changes from the factor XIII, IX, and XI. Based on the confusing results, we thought that decreased activities of factors and prolonged aPTT might partly result from the effects of some ingredients in APL extracts at least. However, we do not know whether the extracts contain some ingredients that causes activation of VII to VIIa, which gives the appearance of increased VII in plasma, and whether the extracts also contain some ingredients that inhibits the activation of VIII, IX, and XI. All above are uncertain, and worth further studying. In summary, we suppose that the macroscopic anticoagulant ability of the APL aqueous extracts may partly base on the greater inhibitory effect on coagulation factor VIII, IX, and XI than that of increasing activity of factor VII.
Platelets are critical for hemostasis both for the formation of blood clots, as platelet aggregates are an essential constituent of the arterial thrombus, and as a platform for activation of coagulation proteins (4). The most important characteristic of GP IIb/IIIa is its affinity modulation of binding to fibrinogen involved in platelet aggregation (26,27). Binding of fibrinogen to the platelet surface can mediate platelet aggregation, and GP IIb-IIIa is an activation dependent receptor for fibrinogen (28). Therefore, FIB-R is formed from glycoprotein IIb/IIIa complex of platelet, and may represent the final common pathway of platelet aggregation (29). In this section, the results indicated that APL aqueous extracts can inhibit platelet aggregation through the inhibition of Fib-R expression to reduce the platelet activation probably involving in blood coagulation. And the results further implies that reduced platelet function may be the important reason of the inhibitory effects of APL aqueous extracts on blood coagulation, and the inhibition of platelet activation may be closely associated with the in vitro anticoagulant and antithrombotic effects of the APL aqueous extracts.
In further study, we found that whole blood and plasma viscosity, as well as IRCA, concentration-dependently increased in the presence of APL aqueous extracts. This disclosed that APL had the ability to elevate blood viscosity, and might accelerate blood coagulation, and the elevated levels of plasma viscosity, and red cell aggregation may be the important causes of elevated blood viscosity in the presence of APL aqueous extracts. The findings revealed that elevated blood viscosity cooperating with increased activity of coagulation factor VII might contribute to the hemostatic effect of APL. Therefore, to some certain extent, the results seemly elucidate the mechanism of hemostasis of APL in part, and they may also provide some experimental supports for this actual use of APL in clinical practice.
There are some limitations concerning our study. Firstly, our study only assessed the in vitro effects of APL aqueous extracts on the major parameters of coagulation function because there were some difficulties to find appropriate animal models to observe the in vivo effects, which could not indicate the in vivo dynamic process of blood coagulation. However, the results also revealed the summary anticoagulant effects of APL extracts. Furthermore, the effects of APL aqueous extracts below 4 g/L are unclear. Therefore, in further study, lower concentrations of the extracts should also be used to find a minimal potent dose. Thirdly, we did not study the effects of APL extracts on biological anticoagulants such as antithrombin, Protein C, S, and TFPI, which might probably reveal the further anticoagulant mechanism of APL extract. In further study, we would perform these experiments to clarify the mechanisms. Finally, APL extracts are the mix of various substances and this study does not identify the specific factors which may influence coagulation. Therefore, we did not know the difference between the effects of the extracts and active ingredients. However, this study is only the preliminary research on the effects of APL on coagulation before further studying active ingredient from the extract.
Conclusions
In conclusion, this study suggests different concentrations of APL aqueous extracts exhibit in vitro opposite pharmacologic effects in blood coagulation, which embodies in the effects of anti-coagulation and pro-coagulation partly through inhibition of intrinsic coagulation pathway and platelet function, and activation of extrinsic coagulation pathway and elevation of plasma viscosity, respectively. These findings indicate that some active constituents derived from APL may be beneficial in the prevention or treatments for abnormal coagulation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81301406), and Provincial Natural Science Foundation of Zhejiang (Grant No. LQ13H190005 and LY17H080007). We thank Dr. Yan Chen of Zhejiang University of Technology for providing APL aqueous extracts and some instructive suggestions in the study period 2014–2015. We thank Dr. QIU Liannv of Zhejiang Provincial Hospital for guiding flow cytometric analysis in the study period 2014-2015.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by local ethics committee on the use of clinical human samples for research of the hospital (ID of ethics approval: 2014KY011), and written informed consent was obtained from all patients.
References
- Abdullah WZ, Moufak SK, Yusof Z, et al. Shortened activated partial thromboplastin time, a hemostatic marker for hypercoagulable state during acute coronary event. Transl Res 2010;155:315-9. [Crossref] [PubMed]
- Wolberg AS, Aleman MM, Leiderman K, et al. Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. Anesth Analg 2012;114:275-85. [Crossref] [PubMed]
- Lipets EN, Ataullakhanov FI. Global assays of hemostasis in the diagnostics of hypercoagulation and evaluation of thrombosis risk. Thromb J 2015;13:4. [Crossref] [PubMed]
- Colman RW. Are hemostasis and thrombosis two sides of the same coin? J Exp Med 2006;203:493-5. [Crossref] [PubMed]
- Lippi G, Salvagno GL, Ippolito L, et al. Shortened activated partial thromboplastin time: causes and management. Blood Coagul Fibrinolysis 2010;21:459-63. [Crossref] [PubMed]
- Lee JJ, Kim T, Cho WK, et al. Antithrombotic and antiplatelet activities of Soshiho-tang extract. BMC Complement Altern Med 2013;13:137. [Crossref] [PubMed]
- Song Y, Yang CJ, Yu K, et al. In vivo Antithrombotic and antiplatelet activities of a quantified Acanthopanax sessiliflorus fruit extract. Chin J Nat Med 2011;9:141-5.
- Park SH, Sim YB, Kang YJ, et al. Effect of Agrimonia pilosa Ledeb Extract on the Antinociception and Mechanisms in Mouse. Korean J Physiol Pharmacol 2012;16:119-23. [Crossref] [PubMed]
- Hong G, Dai YH, Liu PX, et al. Advances in research on chemical constituents and pharmacological activities of Agrimonia pilosa. Pharm. Care & Res 2008;8:362-6.
- Wang JP, Hsu MF, Teng CM. Antihemostatic effect of Hsien-Ho-T'sao (Agrimonia pilosa). Am J Chin Med 1984;12:116-23. [Crossref] [PubMed]
- Shuo LZ, Zhang BS, Zhang RZ. The inhibitory effect of Agrimoni pilosa Ledeb on in vitro thrombosis of rabbits. Chin J Tradit Med Sci Tech 1995;2:21-22.
- Zou XH, Zhang KH, Chen J, et al. Anti-tumor effects of Agrimonia pilosa Ledeb on SMMC-7721 hepatocellular carcinoma cells and its mechanisms. Chungking Med 2013;42:3929-32.
- Fang GZ, Wang HJ, Su WQ. Optimum extracting technology of the druggery composition in Agrimonia pilosa ledeb. J Northeast Forestry Uni 2002;30:36-9.
- Chen G, Fei X, Ling J. The effects of aminoglycoside antibiotics on platelet aggregation and blood coagulation. Clin Appl Thromb Hemost 2012;18:538-41. [Crossref] [PubMed]
- Ma LP, Zhao PR, Wang LX, et al. Inhibitory effects of Agrimonia Pilosa Ledeb water extract on Ecal09 cells in vitro. J Zhengzhou Uni 2007;42:149-51. (Med sci).
- Guzzetta NA, Monitz HG, Fernandez JD, et al. Correlations between activated clotting time values and heparin concentration measurements in young infants undergoing cardiopulmonary bypass. Anesth Analg 2010;111:173-9. [PubMed]
- Fischer F, Appert-Flory A, Jambou D, et al. Evaluation of the automated coagulation analyzer Sysmex CA-7000. Thromb Res 2006;117:721-9. [Crossref] [PubMed]
- Fei XM, Zhou YL, Qiu LN, et al. Inhibition of amikacin on platelet aggregation and blood coagulation. Chin J Lab Med 2010;33:419-24.
- Horton S, Augustin S. Activated clotting time (ACT). Methods Mol Biol 2013;992:155-67. [Crossref] [PubMed]
- Ojito JW, Hannan RL, Burgos MM, et al. Comparison of point-of-care activated clotting time systems utilized in a single pediatric institution. J Extra Corpor Technol 2012;44:15-20. [PubMed]
- Smythe MA, Koerber JM, Nowak SN, et al. Correlation between activated clotting time and activated partial thromboplastin times. Ann Pharmacother 2002;36:7-11. [Crossref] [PubMed]
- van Veen JJ, Spahn DR, Makris M. Routine preoperative coagulation tests: an outdated practice? Br J Anaesth 2011;106:1-3. [Crossref] [PubMed]
- Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol 2007;27:1687-93. [Crossref] [PubMed]
- Gailani D, Renné T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol 2007;27:2507-13. [Crossref] [PubMed]
- Coats TJ, Heron M. Does calcium cause the different effects of Gelofusine and Haemaccel on coagulation? Emerg Med J 2006;23:193-4. [Crossref] [PubMed]
- Joo SJ, Choi JH, Kim SY, et al. An Assay of Measuring Platelet Reactivity Using Monoclonal Antibody against Activated Platelet Glycoprotein IIb/IIIa in Patients Taking Clopidogrel. Korean Circ J 2015;45:378-85. [Crossref] [PubMed]
- George JN. Platelets. Lancet 2000;355:1531-9. [Crossref] [PubMed]
- Schneider DJ. Anti-platelet therapy: glycoprotein IIb-IIIa antagonists. Br J Clin Pharmacol 2011;72:672-82. [Crossref] [PubMed]
- Floyd CN, Ferro A. The platelet fibrinogen receptor: from megakaryocyte to the mortuary. JRSM Cardiovasc Dis 2012.1. [PubMed]


