Nexus between extracellular vesicles, immunomodulation and tissue remodeling: for good or for bad?
Introduction
Over the past three decades or so, the field of immunology has advanced hugely with profound understandings on molecular regulation of immune cells and their contribution to various biological processes. The elaborative tools, in vitro assays, and refined animal models have favorably anticipated the immunomodulatory (immune suppression or activation) mechanisms elicited during the course of disease progression. The projected roles of immune cells are widely attributed to inflammatory diseases, autoimmune diseases, defense against infections, repairing injuries and progression to cancer among others.
Perhaps, the most widespread explanation to activated immune responses is the pattern recognition (1), generally through surface presentation of receptors and antigen presentation prerequisite to communicate direct messages. Antigen presenting cells (APCs), such as dendritic cells (DCs), B cells, macrophages, and mast cells contribute greatly to antigen presentation through major histocompatibility complexes (MHCs) on their surface which is recognized by T cells and favor a cellular cross-talk conferring immune responses (2,3). The immune responses are not exclusively relied on direct cross-talk, however, cells could also extend their messages through secreted trophic factors, such as cytokines, growth factors, transcriptional factors and non-coding RNAs (4), through extracellular vesicles (EVs)—that all may collectively serve as paracrine messengers of cellular cross-talk (5).
EVs are nanosized membrane vesicles (including exosomes and microvesicles) secreted by virtually all cell types including APCs such as B and T lymphocytes, DCs, and mast cells. Interestingly, EVs from APCs contain MHC class I and II, as well as T-cell costimulatory molecules (6-8). EVs have been thought to play unprecedented role in functional transfer of bioactive molecules such as nucleic acids and proteins between cells (9) and enable cell-to-cell communication (10,11). Largely due to their role in intracellular communication they enable, and due to the exchange of bioactive content between cells, EVs have been implicated in the pathogenesis of variety of diseases such as neurodegenerative and cardiovascular disease, immune diseases, and cancer development.
The immunomodulatory and inflammatory roles of EVs have recently been suggested in huge body of evidence (12-15). The most profound aspect of EVs elicited in triggering immune responses or provoking pro-inflammatory responses owe greatly to the presence of MHC-I and -II complexes. This renowned evidence for the first time came from the description of EVs secreted from APCs, for their extended roles to immune responses (6,7). Interestingly, the MHC-complexes carried by DC-derived EVs were capable for the induction of antitumor immune responses in order to facilitate the eradication of tumor cells in in vivo mice models. Such EV-mediated extended functions of APCs, as well as role of EVs in central tolerance, and their contribution to activation or suppression of the immune responses could be exploited for developing prospective immunotherapies (16,17).
The immunomodulatory features of EVs elicited in the context of regenerative processes are scarce in the literature, and are only more recently started being explored (18). Silva et al., in a recent issue published in Eur J Pharm Sci (19), demonstrated the immunomodulatory features of EVs in the context of tissue reparative programs through their ability to participate in immune regulation and inflammation resolution. These features of EVs allow injured tissue to undergo tissue remodeling phase that is perquisite for reparative process. The current study serves as source of valuable knowledge for tissue regenerative biology; however translating this knowledge into therapeutic applications will require deeper understandings on such mechanisms. Moreover, in a certain resident tissue the detrimental effects of EVs conferred through their immunomodulatory properties making EVs good or bad, must also be determined.
This is important to consider that EVs by themselves do not show a uniform molecular pattern; instead they act as conveners and mediators of cellular responses through their cargo shipping ability. This implies that the molecular patterns contained by EVs and the cargo strictly depend on the external conditions, cell state as well as nature and type of the secreting cells which allow immunoreactive or immuno-suppressive consequences in several different ways (Figure 1) (17). Therefore, the immune regulatory features of EVs could be considered in both good and bad. Silva and colleagues propose that knowing the conditions linked to the production of EVs which foster inflammation resolution, could allow manipulation of the inflammatory processes to benefit tissue repair programs (19).
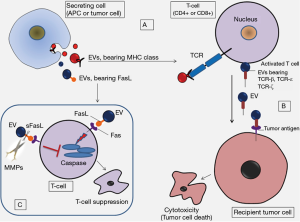
The authors of this study anticipate that EV-mediated transmission of damage-associated molecular patterns to the injury zone could activate certain immune cell populations thereby allowing the onset of the inflammatory response against the injury (19). This proposition supports the idea that the activation of resident immune cells or the homing of additional immune cells at the site of injury/infection could provoke an inflammatory response, which is considered the seeding phase of tissue repair. In fact, tissue repair and regeneration develop along three major phases which are dependent on each other and include stepwise: inflammation resolve → repair → remodeling. During the course of infection or injury the exposed cells of the local tissue get activated that may induce local inflammation. These may include exposed resident epithelial cells, activated immune cells and the fibroblasts that further may promote the recruitment of circulating immune cells as well as growth factors to the site of injury. This reaction is thought to be the first phase in removing pathogens and washing out damaged cells from the injury zone.
The inflammation resolve allows reparative process to progress into the remodeling phase, which is characterized by enhanced angiogenesis facilitated by growth factors, and fibroblast activation. These processes are functionally linked with extracellular matrix (ECM) turnover and the deposition of new ECM, as well as damaged tissue cell replacement that is facilitated by cell proliferation and differentiation. Keeping in view the aforementioned three phases; authors arguably count on the fact that the inflammation resolve is a key phase in the context of tissue repair and regeneration whereby EVs are playing key role. Interestingly, the evolving roles of EVs in tissue repair and regeneration are mainly reliant on their features mimicking stem cell properties and promoting tissue’s intrinsic regenerative programs within recipient cells in a paracrine manner (5). The most profound and relevant therapeutic implications in regenerative medicine that the past two decades have witnessed are those achieved through stem cell assisted tissue regeneration. In this context, stem cell-derived secreted trophic factors such as growth factors, cytokines and EVs could contribute greatly to inducing tissues intrinsic regenerative programs (5). Moreover, the tissue undergoing reparative program requires population equilibrium between cells, which could be accomplished by EV-assisted stem cell proliferation, differentiation and bi-directional communication established between injured tissues and stem cells i.e., injured cells send signals back to stem cells for producing more progenies (5). In this context, authors elaborate that EVs may influence the repopulation of regenerated tissue and functional differentiation of cells. What more can be expected on the beneficial effect of EVs, is their ability to promote angiogenesis—an integral element in healing process, which can be promoted by EV-mediated transportation of pro-angiogenic growth factors to the injury site. Of particular note, the stem cell-derived EVs such as those secreted from mesenchymal stem cells (MSCs) might have immunosuppressive role (16). In this regard Silva et al. envisaged such role of EVs as a major cause of inflammation resolution (19).
The third phase, in the context of repair and regeneration is the ECM turn over and tissue remodeling—that overlaps with above mentioned tissue repair phase. Authors continue to assess the tendency of EVs harboring matrix remodeling molecules which modulate the extracellular environment, as well as matrix deposition at the site of injury (19). One of the class of vesicles known as matrix vesicles have been reported previously for their selective distribution at the sites of initial calcification in cartilage, bone, and predentin and are thought to have role in mineralizing of vertebrate tissues during bone development (20). This indicates the importance of calcification and mineralization in developing matrix. Considering the fibroblasts activity in ECM environment, it is also notable that fibroblasts could be differentiated into cancer associated fibroblasts (CAFs) [reviewed elsewhere (16)]. However, despite the evidences for the involvement of EVs in ECM degradation, the relevance of their enzymatic activity in ECM turnover during tissue repair has not been fully explored.
Discrepancy of EVs in eliciting immune responses and immunotherapy
Hitherto, the concept of EV-mediated immune exploitation of target cells is extremely attractive, nevertheless several questions of such process requires critical considerations. The most important consideration would be to determine the relationship between two different aspects of immune responses such as immunosuppressive features versus immune provoking potentials of EVs which depend on several factors (Box 1). These discrepancies may represent EVs with variable outcomes in therapeutic perspectives. Presumably, EV-mediated overwhelming immune activation and pro-inflammatory cascades occurring at the site of injury may have undesirable and devastated effects. An example could be seen in the down-regulation of NK and B-cell proliferation by inflammatory cytokines (21). It is anticipated that a pro-inflammatory environment could not only modify the composition of EVs but also the consequent biological activities of immune effector cells, with possibility of increased risks of unpredictable effects (14).

Full table
Tissue regeneration therapy generally requires an immunosuppressive environment, in particular during organ implants; whereas the cancer immunotherapy largely relies on evoking the host immune system to fight against cancer cells (16). This reflects that for the purpose of repair programs the EV-mediated immune responses will need to be manipulated differently from those manipulated for the purpose of tumor eradication. The immunosuppressive tumor microenvironment is considered a major barrier to the effectiveness of anti-tumor immune activities, since it offers lower immunogenicity of immune cells against the cancer cells. However, growing literature on EVs functional roles continue to provide us with new insights in understanding such discrepancies.
Other therapeutic applications of EVs
In parallel to other beneficial effects resulted from transport of bioactive molecules and intercellular communication—EVs could also be applied as drug delivery vehicles. This is largely due to their natural tendency to transport biological molecules as well as their biocompatibility with the target cells. In the context of drug delivery vehicles, a relatively different but potentiating proposition of EVs—is their pharmacokinetics and pharmacodynamics (19), which could be tailored for pharmaceutical purposes in in vivo animal studies.
However, there remain several potent issues to be solved. For instance, loading efficacy and stability of a certain drug is a major concern, as has been observed with other delivery vectors. The additional consideration is the specific targeting issues, since EVs having surface chemistries compatible with cell receptors, could interact with unpredicted cells/tissues that may give undesired results/effects. Moreover, donor cell derived EV-cargo could provoke immune responses in recipient cells with a possibility to confer cross-reactivity.
Considering these facts, one of the important aspect of the description by Silva and colleagues could be in vivo administration of EVs, biodistribution and the delivery of EV-cargo to targeted destinations (19). However, the targeted uptake and internalization of EVs by proposed target recipient cells remains an impeding question. Some of the strong clues provided by Hoshino et al., offer interesting information with the arguments that EVs could seek target organs through different forms of surface integrin’s presented on their surface (22). This knowledge could guide researchers for in vivo delivery of EV-loaded drugs, however, further studies will warrant translating this knowledge into targeted and organ guided drug delivery.
From bench to bedside
Pertaining to therapeutic applications in the context of tissue regeneration—the feasibility of EV-based therapies have not been eventuated in clinical trials (19). However, there is initial evidence for applying EVs to tissue healing process in an individual patient case. Kordelas et al. showed that MSC-derived EVs are well tolerated in patients during the treatment of graft-versus-host disease (GVHD) (23). Moreover, MSC-derived EVs treatment significantly reduced the pro-inflammatory cytokine response in patients’ peripheral blood mononuclear cells (PBMCs) in vitro, as well as the clinical symptoms of GVHD were improved significantly shortly after the start of MSC-derived EV therapy to the patient (23). It was proposed that the donor derived EVs could recapitulate the immunomodulatory properties of MSCs. Therefore, the applications of immunosuppressive EVs could be of great therapeutic value for future clinical consideration due to the fact that such EVs are well tolerated in patients.
Despite improvements, both in the clinical procedures intended for tissue repairs, organ transplantation and cell-based therapies over the last decade, current methods present potential complications (for example, an increased risk of infection, toxicity, and graft rejection). In this context, compared with traditional stem cell therapies, EV-based cell-free therapies may improve patients’ outcomes considerably with reduced complications as compared to cell-based therapy (16). However, stem cell-based therapies also need to consider potent risk factors and technical complications such as: culture-induced senescence, genetic instability, loss of functional properties, immune-mediated rejection, and the risk of transformation of resident cells into malignant phenotypes which presumably limit the applications of stem cells in tissue regeneration (5). Therefore, steering traditional stem cell-based therapy toward EV-based therapy is still a debated issue. In this regard, this could be of interest to applying combination of EV-based therapies with existing approaches in order to improve the therapeutic benefits.
Parallel to clinical trials on tissue regeneration, the evaluation of EVs for clinical trials in other human diseases is also very limited (24,25). However, this interesting to consider that in spite of very small number of patients included in these clinical trials, yet the potential of EVs for their prospective translation from bench to bedside is thought be promising. However, several technical hurdles still require an explicit attention. A potential challenge in the field exists largely due to the limitations of standardizing the existing technologies. In particular, standard protocols for EV isolation, purification and characterization are still a debated issue (9,26). It has been argued that the development of high-throughput approaches and robust capture platforms will warrant the implications of EVs in routine biomarker development, and therapeutic implications with a proposed workflow sheet to applying for USA food and drug administration (FDA) approval (27). Since there is intensive interest in the field both in basic research as well as therapeutic point of view—it is anticipated that in the next decade, EVs arena will see significant advances in clinical pipelines.
Acknowledgements
Authors acknowledge the FAPESP (Sao Paulo Research Foundation, Proc. No. 12/24574-3) and CAPES (coordination of the higher education personal, Proc. No. BEX 7057/15-6 and (Proc. No. BEX 6332/15-3) in Brazil.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brubaker SW, Bonham KS, Zanoni I, et al. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 2015;33:257-90. [Crossref] [PubMed]
- Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol 2014;14:719-30. [Crossref] [PubMed]
- den Haan JM, Arens R, van Zelm MC. The activation of the adaptive immune system: cross-talk between antigen-presenting cells, T cells and B cells. Immunol Lett 2014;162:103-12. [Crossref] [PubMed]
- Fatima F, Nawaz M. Vesiculated Long Non-Coding RNAs: Offshore Packages Deciphering Trans-Regulation between Cells, Cancer Progression and Resistance to Therapies. Non-Coding RNA 2017;3:10. [Crossref]
- Nawaz M, Fatima F, Vallabhaneni KC, et al. Extracellular Vesicles: Evolving Factors in Stem Cell Biology. Stem Cells Int 2016;2016:1073140.
- Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996;183:1161-72. [Crossref] [PubMed]
- Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998;4:594-600. [Crossref] [PubMed]
- Théry C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 1999;147:599-610. [Crossref] [PubMed]
- Mateescu B, Kowal EJ, van Balkom BW, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA – an ISEV position paper. J Extracell Vesicles 2017;6:1286095. [Crossref] [PubMed]
- Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006;20:847-56. [Crossref] [PubMed]
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [Crossref] [PubMed]
- Buzas EI, György B, Nagy G, et al. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol 2014;10:356-64. [Crossref] [PubMed]
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014;14:195-208. [Crossref] [PubMed]
- Burrello J, Monticone S, Gai C, et al. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front Cell Dev Biol 2016;4:83. [Crossref] [PubMed]
- Greening DW, Gopal SK, Xu R, et al. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol 2015;40:72-81. [Crossref] [PubMed]
- Fatima F, Nawaz M. Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chin J Cancer 2015;34:541-53. [Crossref] [PubMed]
- Nawaz M, Fatima F, Nazarenko I, et al. Extracellular vesicles in ovarian cancer: applications to tumor biology, immunotherapy and biomarker discovery. Expert Rev Proteomics 2016;13:395-409. [Crossref] [PubMed]
- Withrow J, Murphy C, Liu Y, et al. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther 2016;18:286. [Crossref] [PubMed]
- Silva AM, Teixeira JH, Almeida MI, et al. Extracellular Vesicles: Immunomodulatory messengers in the context of tissue repair/regeneration. Eur J Pharm Sci 2017;98:86-95. [Crossref] [PubMed]
- Shapiro IM, Landis WJ, Risbud MV. Matrix vesicles: Are they anchored exosomes? Bone 2015;79:29-36. [Crossref] [PubMed]
- Di Trapani M, Bassi G, Midolo M, et al. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep 2016;6:24120. [Crossref] [PubMed]
- Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527:329-35. [Crossref] [PubMed]
- Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014;28:970-3. [PubMed]
- Fais S, O'Driscoll L, Borras FE, et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano 2016;10:3886-99. [Crossref] [PubMed]
- Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 2015;4:30087. [Crossref] [PubMed]
- Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013.2. [PubMed]
- Nawaz M, Camussi G, Valadi H, et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat Rev Urol 2014;11:688-701. [Crossref] [PubMed]


