A radiation-induced and radiation-sensitive, delayed onset angiosarcoma arising in a precursor lymphangioendothelioma
Introduction
The application of radiotherapy in the treatment of breast carcinoma has become standard practice due to its recognizable benefits in the reduction in the risk of loco-regional cancer recurrence and in both subsequent morbidity and mortality (1,2). Following radiation exposure for breast cancer, there are a number of cutaneous complications that may develop in the immediate and long term, one of the most feared being secondary neoplasia. Although rare in the context of breast cancer, these may include secondary breast and lung carcinomas, malignant fibrous histiocytomas, osteosarcomas and fibrosarcomas (3,4).
Angiosarcomas are one histological subtype of radiation-induced sarcomas, and although a very rare complication, they are significant due to their poor overall prognosis (5-7). Characterised by rapid proliferation and extensive infiltrating growth, these malignant tumours of the vascular endothelium typically follow an aggressive course with difficulty clearing surgical margins and controlling distant spread. They are typically poorly responsive to radiotherapy and chemotherapy (6,8).
We present the case of an 83-year-old female patient previously treated with radiotherapy for a left breast carcinoma, who presented 38 years later with a large and uncharacteristically radio-sensitive angiosarcoma of the left upper back.
Case presentation
An 83-year-old female of Eastern European background presented with a large angiosarcoma of the left upper back within a previously irradiated field, approximately 38 years after undergoing treatment for a left breast carcinoma. Following diagnosis of an early left breast carcinoma the patient underwent a mastectomy and left axillary lymph node clearance followed by radiotherapy, probably five-field (chest wall, supraclavicular fossa and axilla) in New Zealand in 1970. The patient’s radiotherapy records were unable to be retrieved, however post-radiation changes (poikiloderma and telangiectases) clinically apparent on the chest wall confirmed that she received radiotherapy to this area and most likely, also to the supraclavicular fossa. The patient likely also received radiotherapy to the axilla, given that she had chronic moderate lymphoedema of the ipsilateral arm. Her clinical course was without evidence of further treatment complications or tumour recurrence.
In the late 1990’s, approximately 30 years later, the patient first noticed a small patch of thickening and scaliness on the left upper back. Serial excision biopsies revealed a lymphangioendothelioma with no suggestion of malignancy. According to the patient, in early 2006 the lesion began to enlarge and take on a more erythematous plaque-like appearance. She was reviewed by a specialist dermatologist and a further excision biopsy revealed a low-grade cutaneous angiosarcoma (i.e., approximately 38 years post initial breast cancer therapy), extending to the level of the mid-dermis. Clinically, the lesion on the left upper back was erythematous, ulcerated and slightly raised with a plaque-like appearance with extensions (with some areas of discontinuity) representing disease superiorly to the supra-clavicular fossa and inferiorly to approximately the T6 vertebral level. The lesion now extended both above and below what would have been the previous supraclavicular fossa radiotherapy exit field.
The patient was subsequently referred to a general surgeon who performed excision of the lesion with successful skin grafting. Pathology showed an apparently complete excision. This was followed by further complete pathological excisions of two newly-appearing cutaneous nodules lateral to the graft approximately four months later. Wide-field post-operative radiotherapy was delivered to the previously involved regions to a dose of 60 Gy in 30 fractions in 2007. She tolerated the radiotherapy well. One year later there was progressive disease around the anterior aspect of the left neck, tracking into the posterior triangle. There was no recurrence around the original graft posteriorly or laterally, and no visceral metastases evident on restaging CT. The angiosarcoma continued to progress within the posterior triangle of the left neck with areas of extension down the left chest wall and also within and beyond the skin graft on the back (Figures 1,2). Palliative radiotherapy of 10 Gy in 2 fractions was delivered to the regions of macroscopic disease on the back, using a direct 12 MeV electron beam. Surprisingly, there was a complete clinical response to this low dose radiotherapy. Two months later the patient received a further 8 Gy in 2 fractions to the anterior chest wall using a 9 MeV electron beam, again with a complete clinical response. Her excellent response to low-dose radiotherapy was highly uncharacteristic of this tumour. Unfortunately, she continued to develop new areas of active disease outside the treatment margins. The patient was referred to a medical oncologist for consideration of systemic treatment. She received 4 months of weekly Taxol, however due to side effects she requested treatment be terminated. She died 4 months later of progressive locoregional and hepatic metastatic disease.
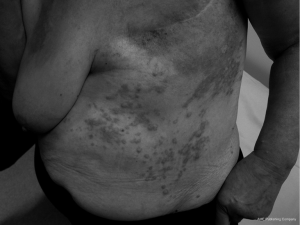
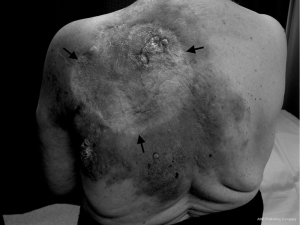
Discussion
The first recorded description of angiosarcoma development post radiotherapy was in the anterior abdominal wall of a 66-year-old man irradiated 6 years previously for a penile cancer (9). Following this, a number of cases of chest wall angiosarcoma were described in the literature in women post mastectomy and radiotherapy [reviewed in (5)].
Angiosarcoma of the breast arise in three different clinical settings: primary spontaneous angiosarcoma, secondary radiation-induced angiosarcoma, and Stewart-Treves Syndrome (STS) (10,11). Primary angiosarcomas of the breast generally occur between the ages of 20–40 years and are rare, with an incidence of less than 0.0005% (10). These constitute less than 0.01% of all breast cancers (4,5). Following review of published cases on the subject, Abbott and Palmieri (5) noted that secondary angiosarcomas following mastectomy and radiotherapy presented at a median age of 67 years with a median latency of 72 months between radiotherapy and angiosarcoma development. They also noted the majority of these women received a radiation dose of over 40 Gy. These secondary angiosarcomas occurring post-radiotherapy have three defining characteristics: histological distinction from the primary neoplasm, location/origin within the previously irradiated field, and latency of at least a number of years following radiotherapy. Our case had a latency of 38 years post-therapy, the previously reported longest latency period being 16 years (5). STS describes development of an angiosarcoma in the setting of chronic upper limb lymphoedema following mastectomy and axillary dissection. It is estimated that STS occurs in approximately 0.07% of patients following axillary dissection, and develops approximately 10 years after surgery (12,13); it is likely that this frequency may be declining due to improvements in surgical techniques and trends towards minimalization of axillary surgery in breast cancer.
Our patient developed a lymphangioendothelioma on her back within the presumed previous radiotherapy exit field; approximately 8 years later this possible precursor lesion in the same area appeared to progress to an angiosarcoma. While it cannot be known with certainty, it is likely that this was a precursor lesion to the angiosarcoma; if so, this would be the first post-therapy angiosarcoma rising from a documented precursor lesion—angiosarcomas mostly develop de novo. This was now approximately 38 years after her previous radiotherapy treatment. While this represents a much longer latency period than the median previously described in the literature, the case of angiosarcoma presented here fits the criteria for radiation induced angiosarcoma (see above).
There is currently no clinical data regarding the optimum treatment for post radiotherapy angiosarcomas (5). Treatment modalities include surgery, most commonly used, along with radiotherapy, and/or chemotherapy. In the case of our patient, the rapid chest wall recurrence outside the treatment margins confirmed this as a typically aggressive tumour; however this tumour was uncharacteristically radiosensitive, in that low-dose palliative radiotherapy resulted in rapid and complete clinical responses within the irradiated areas. This is contrary to the usual poor responses of angiosarcoma to radiotherapy (6,8).
Regarding her systemic therapy, Taxol, as used here, is a standard treatment (14).
However, given her unusual tumour sensitivity to radiotherapy, radiomimetic agents such as bleomycin may have also shown some clinical utility. In addition, drugs targeting vascular endothelial growth factor receptors (VEGFR) have been considered in this disease. Vascular endothelial growth factor (VEGF) is a potent mediator of angiogenesis, and thus may play an important role in the development and progression of angiosarcoma. Studies have suggested involvement of the VEGFR system in the growth and proliferation of hemangiosarcoma cells in vitro (5). Furthermore, it has been reported that positive immunohistochemical staining for VEGF occurs in 14% of primary angiosarcomas and of its 3 known receptors, VEGFR3 has been shown to be present in 50% of all angiosarcomas (5). Given the potential involvement of these signalling pathways in the pathogenesis of post-radiotherapy angiosarcoma, it seems reasonable to consider the use of VEGF antagonists such as bevacizumab (humanized monoclonal antibody targeting VEGF-A), sorafenib (an inhibitor of VEGFR1, VEGFR2, VEGRF3 among other receptors) (15), pazopanib (an inhibitor of VEGF2 and VEGF3) (16,17), apatinib (an inhibitor of VEGF2) (18) or even thalidomide (known to suppress VEGF) (19) in the treatment of angiosarcoma.
Conclusions
We have discussed the case of an 83-year-old lady who developed an angiosarcoma following radiotherapy for a left breast carcinoma 38 years earlier. The presence of a possible precursor lesion could suggest that lymphangioendotheliomas should be excised widely, in case they may progress to malignancy. This angiosarcoma was uncharacteristically radio-sensitive, and although angiosarcoma is usually considered radiation-resistant, raises the possibility that initial radiotherapy for these lesions should be considered, for example, as a routine up-front surgical adjuvant. Further, the biology of these cancers may suggest that antagonists or suppressors of VEGF pathways may have clinical utility in their treatment.
Clinical practice points
Here we describe a patient with a probable post-radiotherapy angiosarcoma of the chest wall after previous breast cancer therapy. Post-radiotherapy chest wall angiosarcomas are well-described, albeit at a very low frequency. Angiosarcomas are typically radioresistant tumours. This case is notable for three reasons. First, it was highly sensitive to low-dose palliative radiotherapy. This raises a potential rationale for greater integration of radiotherapy into breast/chest wall angiosarcoma combination therapy. Second, it was likely that this patient’s angiosarcoma arose from a precursor lesion (lymphangioendothelioma), to our knowledge the first such description, since post-radiotherapy chest wall angiosarcomas usually arise de novo. This suggests that lymphangioendotheliomas should be treated with caution, perhaps with greater than currently employed resection margins. Third, the interval to development of the post-radiotherapy angiosarcoma was the longest described in the literature, 38 years, suggesting that clinicians should remain wary of new radiotherapy in-field skin lesions for the duration of a patient’s lifetime after breast cancer therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics approval (ID: 8-112) for this study, employing cell lines from cancer patients, was obtained from the Ethics Committee of the Peter MacCallum Cancer Centre, Melbourne, Australia. Consent was obtained from the patient for publication of this case report and any accompanying images.
References
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Uchin JM, Billings SD. Radiotherapy-associated atypical vascular lesions of the breast. J Cutan Pathol 2009;36:87-8. [Crossref] [PubMed]
- Rao J, Dekoven JG, Beatty JD, et al. Cutaneous angiosarcoma as a delayed complication of radiation therapy for carcinoma of the breast. J Am Acad Dermatol 2003;49:532-8. [Crossref] [PubMed]
- Abbott R, Palmieri C. Angiosarcoma of the breast following surgery and radiotherapy for breast cancer. Nat Clin Pract Oncol 2008;5:727-36. [Crossref] [PubMed]
- Wang XY, Jakowski J, Tawfik OW, et al. Angiosarcoma of the breast: a clinicopathologic analysis of cases from the last 10 years. Ann Diagn Pathol 2009;13:147-50. [Crossref] [PubMed]
- Fodor J, Orosz Z, Szabó E, et al. Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol 2006;54:499-504. [Crossref] [PubMed]
- Arbiser JL, Bonner MY, Berrios RL. Hemangiomas, angiosarcomas, and vascular malformations represent the signaling abnormalities of pathogenic angiogenesis. Curr Mol Med 2009;9:929-34. [Crossref] [PubMed]
- Calnan J, Cowdell RH. Lymphangio-endothelioma of the anterior abdominal wall; report of a case. Br J Surg 1959;46:375-9. [Crossref] [PubMed]
- Buatti JM, Harari PM, Leigh BR, et al. Radiation-induced angiosarcoma of the breast. Case report and review of the literature. Am J Clin Oncol 1994;17:444-7. [Crossref] [PubMed]
- Mark RJ, Poen J, Tran LM, et al. Postirradiation sarcomas. A single-institution study and review of the literature. Cancer 1994;73:2653-62. [Crossref] [PubMed]
- Wysocki WM, Komorowski A. Stewart-Treves syndrome. J Am Coll Surg 2007;205:194-5; author reply 195. [Crossref] [PubMed]
- Strobbe LJ, Peterse HL, van Tinteren H, et al. Angiosarcoma of the breast after conservation therapy for invasive cancer, the incidence and outcome. An unforseen sequela. Breast Cancer Res Treat 1998;47:101-9. [Crossref] [PubMed]
- Nagano T, Yamada Y, Ikeda T, et al. Docetaxel: a therapeutic option in the treatment of cutaneous angiosarcoma: report of 9 patients. Cancer 2007;110:648-51. [Crossref] [PubMed]
- Penel N, Ray-Coquard I, Bal-Mahieu C, et al. Low level of baseline circulating VEGF-A is associated with better outcome in patients with vascular sarcomas receiving sorafenib: an ancillary study from a phase II trial. Target Oncol 2014;9:273-7. [Crossref] [PubMed]
- Ogata D, Yanagisawa H, Suzuki K, et al. Pazopanib treatment slows progression and stabilizes disease in patients with taxane-resistant cutaneous angiosarcoma. Med Oncol 2016;33:116. [Crossref] [PubMed]
- Ravi V, Sanford EM, Wang WL, et al. Antitumor Response of VEGFR2- and VEGFR3-Amplified Angiosarcoma to Pazopanib. J Natl Compr Canc Netw 2016;14:499-502. [PubMed]
- Ji G, Hong L, Yang P. Successful treatment of angiosarcoma of the scalp with apatinib: a case report. Onco Targets Ther 2016;9:4989-92. [Crossref] [PubMed]
- Raina V, Sengar M, Shukla NK, et al. Complete response from thalidomide in angiosarcoma after treatment of breast cancer. J Clin Oncol 2007;25:900-1. [Crossref] [PubMed]


