Targeting the seeds of small cell lung cancer
The past decade has ushered the new discipline of “Translational Medicine” across academic medical research, the pharmaceutical industry and the regulatory agencies. The term, “translational medicine” per-se often needs “translation” in respect to what it means along the numerous practices that ultimately transform science into effective and safe drugs. The greatest of challenges in translational medicine has been encountered in oncology, where imprecise pre-clinical models and hurdles such as tumoral heterogeneity have impeded progress in advancing targeted therapies into clinical trials (1). In recent years, the advent of patient derived xenograft (PDX) models combined with sophisticated genomic technologies (i.e., next-generation sequencing) have ushered a new era where cancer targets are identified within the appropriate biological framework and validated using patient derived and clinically relevant cancer models.
The report by Saunders and colleagues featured in Science Translational Medicine is well embedded in this path (2). Utilizing transcriptomic profiles in small cell and large cell neuroendocrine carcinoma (LCNEC) PDX and patient samples, the authors identified significant overexpression (>100-fold) of the Notch ligand Delta like 3 (DLL3) compared to normal tissues of lung and other vital organs. While DLL3 shares significant homology with other Notch family ligands, its reported localization to the Golgi apparatus suggests it does not participate in Notch pathway activation (3). Importantly, the authors developed a DLL3 specific monoclonal antibody and showed that in the context of neuroendocrine lung cancers, at least some DLL3 does localize to the cell surface. The presence of cell surface DLL3, coupled with its specific overexpression in cancer cells, suggests that it may serve as a bona fide target for an antibody drug conjugate (ADC).
ADCs as a therapeutic platform
ADCs are a powerful means of specifically delivering cytotoxins to cancer cells via targets expressed at the cell surface (4). Like a Trojan horse, internalization of the ADC leads to release of the cytotoxic molecule and rapid cancer cell death. Important criteria for the clinical efficacy of ADC based therapies are both the absolute level of cell surface expression of the target and its relative expression compared to normal tissues. Underscoring this, while many ADCs have entered clinical phase trials, only two are currently FDA approved, Trastuzumab emtansine (targeting HER2; Genentech) (5) and Brentuximab vedotin (targeting CD30; Millenium/Takeda) (6). It is important to note that in both cases, the targets (CD30 and HER2) are highly overexpressed at the cell surface of cancer cells. In the case of HER2, 100–200 fold above what is seen in normal tissues (7). The high level of DLL3 overexpression seen in neuroendocrine-derived lung cancers compared to normal tissues (100 times) is certainly within this range and suggests great promise for DLL3 as an ADC target. Indeed, DLL3 expression is found in most small cell tumor cells and correlated with high levels of ADC efficacy in debulking DLL3 positive PDX models. Of note, previous studies have not reported cell surface expression of DLL3, therefore major questions remain as to whether the cell surface expression pattern is unique to neuroendocrine cancers of the lung or is seen in other normal cells at low levels. As DLL3 is also expressed in the cytoplasm, localization at the membrane is difficult to appreciate by immunohistochemical techniques. Thus, a more detailed analysis of normal tissue expression by flow cytometry would be helpful in this regard. Cleary, the lack of activity of a naked DLL3 neutralizing antibody on DLL3 positive lung cancer PDX models indicates that DLL3 cell surface expression is not a cancer driver in these tumors. Further, the observed tolerability of the DLL3 ADC in preclinical rat and cynomolgus monkey models certainly mitigates this possibility and provides further evidence that a safe therapeutic window may be achieved in patients. Thus, this paper presents compelling evidence that an ADC targeting of DLL3 positive tumors should be explored in the clinical setting.
Targeting the tumor initiating cancer cell
The heterogeneous nature of mutations and gene expression programs in tumors makes them difficult to target in many respect. Thus, it is no surprise that homogenous target expression has been generally an important criterion for advancing ADC targets through the pre-clinical pipeline. With regards to the majority of ADC targets, which are not true cancer drivers, this may be difficult to achieve as there is no positive selection for their expression within the tumoral milieu. In general, the importance of consistent target expression has correlated well to ADC activity in a number of cases (8). Nevertheless, in certain cases, ADCs have also demonstrated efficacy even in tumors with variable expression (8). Two plausible scenarios may explain this benefit. (I) A bystander effect kills the non-target expressing cancer cells when free drug is released upon cancer cell death and (II) The target expressing cells are intrinsically more important for maintaining the tumorigenic properties of the cancer.
Indeed, the observed intratumoral heterogeneity in many cancer types has led to intense investigation as to whether sub-populations of cells within the tumor are more tumorigenic than others (9). A number of groups have shown that infrequently found stem-like cells within the tumor are more efficient at forming tumors and are recalcitrant to standard of care (SOC) chemotherapeutics. Thus, it has been postulated that if one can specifically target these tumor-initiating cancer cells (TICs), greater therapeutic efficacy will be achieved, particularly in 2nd or 3rd line chemotherapy relapsed patients. Indeed, the authors showed that targeting DLL3 positive tumor cells significantly reduces the frequency of TICs compared to vehicle or SOC chemotherapy. Consistent with these findings, Saunders and colleagues found that dosing of DLL3 ADC in innate or acquired chemoresistant PDX models, leads to durable responses in vivo.
Recent studies appearing in the last year in Science Translational Medicine and Nature have suggested similar effects of treating the stem cell compartment in colorectal cancer. These studies emanating from Genentech Inc. showed that Wnt pathway addicted colorectal cancers can be efficiently targeted using either a LGR5 targeted ADCs or a RSPO3 neutralizing naked antibody (10,11). Cumulatively, these studies are consistent with an intriguing yet controversial theory termed the cancer stem cell hypothesis that suggests TICs as the main drivers and therapeutic targets of relapsed disease (Figure 1). Consistent with a potential stem like function of these TICs, de Sauvage and colleagues found that Wnt pathway inhibition leads to durable response associated with a differentiation phenotype (11). Moreover, evidence was presented to suggest that these effects are due to specific killing of a TIC compartment that is marked by intestinal stem cell genes. While, Saunders and colleagues did not examine differentiation as an endpoint per se, the association between DLL3 and the lineage transcription factor ASCL1 and the previous association between Notch signaling and neuroendocrine lineage commitment would make the DLL3 targeted agents acting as a differentiation therapy an intriguing possibility.
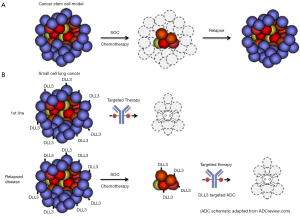
Translating preclinical success to the clinic
A dearth of targeted therapies highlights an unmet medical need for colorectal cancer and small cell lung cancer patients. This underscores the importance of efficiently translating these recent pre-clinical successes of TIC targeted ADCs into the clinic. Major hurdles that have hindered a number of ADC programs in this regard include safety/tolerability issues, limited depth of pre-clinical modeling, and differences in pharmacodynamics/pharmacokinetic (PK/PD) scaling between rodent models to human. While we await clinical data on LGR5 and RSPO3 targeted therapeutics, preliminary data presented at ASCO in June 2016 showed early promise for the DLL3 targeted ADC (12). Indeed, a Phase I clinical trial of the DLL3 targeted ADC (rovalpituzumab tesirine) showed measurable response in small cell lung cancer patients. Patients with the highest level of DLL3 showed significant tumor shrinkage. However, these results were tempered by an increase in median overall survival (5.8 months in the rovalpituzumab tesirine treated group). While promising, the modest increase in overall survival raises question as to the durability of the response and whether this ADC is targeting TICs. Thus, further studies in larger patient cohorts will be necessary to determine the clinical benefit of targeting DLL3 in neuroendocrine tumors and whether this benefit is derived from inhibiting TIC activity and tumor regrowth.
In summary, ADCs targeting the TIC compartment of tumors is an exciting concept, combining time-tested chemotherapeutics with precision medicine approaches. The reports in STM by Saunders and Junttila et al. are important studies that lay the conceptual foundation for targeting TICs in cancer. The next few years surely will present critical data that will enhance our understanding of ADC activity in targeting these distinct cancer sub-populations in the clinical setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dienstmann R, Rodon J, Barretina J, et al. Genomic medicine frontier in human solid tumors: prospects and challenges. J Clin Oncol 2013;31:1874-84. [Crossref] [PubMed]
- Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7:302ra136. [Crossref] [PubMed]
- Geffers I, Serth K, Chapman G, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol 2007;178:465-76. [Crossref] [PubMed]
- Tolcher AW. Antibody drug conjugates: lessons from 20 years of clinical experience. Ann Oncol 2016;27:2168-72. [Crossref] [PubMed]
- Burris HA 3rd, Tibbitts J, Holden SN, et al. Trastuzumab emtansine (T-DM1): a novel agent for targeting HER2+ breast cancer. Clin Breast Cancer 2011;11:275-82. [Crossref] [PubMed]
- Alperovich A, Younes A. Targeting CD30 Using Brentuximab Vedotin in the Treatment of Hodgkin Lymphoma. Cancer J 2016;22:23-6. [Crossref] [PubMed]
- Ram S, Kim D, Ober RJ, et al. The level of HER2 expression is a predictor of antibody-HER2 trafficking behavior in cancer cells. MAbs 2014;6:1211-9. [Crossref] [PubMed]
- Asundi J, Crocker L, Tremayne J, et al. An Antibody-Drug Conjugate Directed against Lymphocyte Antigen 6 Complex, Locus E (LY6E) Provides Robust Tumor Killing in a Wide Range of Solid Tumor Malignancies. Clin Cancer Res 2015;21:3252-62. [Crossref] [PubMed]
- Shukla G, Srivastava AK, Patidar R, et al. Therapeutic Potential, Future Perspective and Challenges of Cancer Stem Cells in Translational Oncology: A Critical Review. Curr Stem Cell Res Ther 2017;12:207-24. [Crossref] [PubMed]
- Dashti SG, Chau R, Ouakrim DA, et al. Female Hormonal Factors and the Risk of Endometrial Cancer in Lynch Syndrome. JAMA 2015;314:61-71. [Crossref] [PubMed]
- Storm EE, Durinck S, Tremayne J, et al. Targeted therapy directed against PTPRK-RSPO3 fusion-positive colorectal tumours inhibit stem cell function and promote differentiation. Nature 2016;529:97-100. [Crossref] [PubMed]
- Goodman A. Impressive Early Data for Rovalpituzumab Tesirine in Small Cell Lung Cancer. 2016. Available online: http://www.ascopost.com/issues/june-25-2016/impressive-early-data-for-rovalpituzumab-tesirine-in-small-cell-lung-cancer/


