Fibrosis assessment in patients with chronic hepatitis B virus (HBV) infection
Introduction
Worldwide, more than 2,000 million people have been infected with hepatitis B virus (HBV) during their lifetime (1). Of these, about 350 million remain chronically infected (CHB). Three-quarters of the world’s population live in areas with high levels of infection. An estimated 1 million people die each year from HBV-related cirrhosis or primary liver cancer (1). HBV has a complex natural history, centred in the liver, where the interaction between viral proteins and the immune system leads to a cycle of hepatocyte damage and tissue repair (2). This repair involves the repeated deposition of extracellular matrix leading to progressive liver fibrosis over time. The HBV X protein may also have particular fibrogenic and oncogenic effects on liver (3). Progression to advanced fibrosis can be rapid, slow, or sporadic depending on disease state and the degree of active liver inflammation and injury. The formal assessment of liver fibrosis is vital to disease prognostification, and to determine the urgency of treatment as well as the response to therapy. The major predictor of outcome is the severity of liver disease at presentation. Cirrhosis is associated with reduced survival and an increased incidence of HCC (4,5). Cirrhosis is associated with 5- and 20-year survival rates of 55% and 25%, respectively, whereas these rates are 97% and 63%, respectively for patients without cirrhosis (6). The presence of advanced fibrosis on non-invasive assessment is an independent predictor of HCC development (7). Although traditional blood tests such as alanine aminotransferase (ALT) levels are useful as measures of disease activity, they have proven poor indicators of liver fibrosis alone (8). Studies in Asia and the United States revealed that 20% to 30% of HBV carriers with persistently normal ALT levels and HBV DNA levels >104 copies/mL have stage ≥2 inflammation and stage ≥2 fibrosis on liver biopsy (9). Hence, fibrosis assessment by either liver biopsy or dedicated noninvasive tests can aid prognostication of these individuals. Furthermore, direct assessment of fibrosis is recommended to clarify indeterminate cases where there is a discordance between ALT and HBV DNA levels in order to guide treatment decisions (10-12). More recently, studies have shown that sustained HBV suppression with antiviral treatment can lead to a reduction in necroinflammatory activity and improvement in fibrosis stage, including the regression of cirrhosis in some (13). Non-invasive markers are useful adjuncts to demonstrate the effects of treatment without the need for repeated liver biopsies (14). A plethora of non-invasive tests have been developed in recent years in an attempt to reduce the need for liver biopsy and to better inform clinical practice (15,16). This review describes the current modalities for assessment of fibrosis in HBV, including the role of liver biopsy as well as blood and imaging-based non-invasive markers in disease management.
Liver biopsy
In HBV infection, liver biopsy is the gold standard for assessing the degree of liver injury, including both inflammatory activity and fibrosis stage (17). Additionally, a liver biopsy may be used to confirm hepatocellular carcinoma (HCC) or identify the co-existence of other diseases. In HBV, there is a varying degree of predominantly lymphocytic portal inflammation with interface hepatitis and spotty lobular inflammation. Inflammation is minimal in the immune-tolerant and inactive carrier phases but is pronounced in the immune reactive phase. Bridging necrosis and confluent necrosis can be seen (17). Knodell, Ishak, and METAVIR systems are the histological systems more routinely used to assess the disease activity and treatment response. The goal of treatment is to stop ongoing necroinflammation and prevent fibrosis progression (18). Fibrosis stage is relevant for both histological prognostication and treatment initiation. In the traditional histological scoring systems, the histological score does not relate to the amount of fibrosis (19). Instead, histological scoring is based on the subjective visual interpretation of architectural changes of fibrosis, without quantifying fibrosis as a continuous variable, but rather report as a semi-quantitative numerical stage. These numbers are not arithmetically proportionate, i.e., stage 2 is not half of stage 4 (19,20). The need for an objective method, has led to the increasing use of digital image analysis (DIA) technology with collagen quantification using collagen proportionate area (CPA) for liver fibrosis assessment (21-25). As the number of hepatocytes decreases with increasing number of collagen deposition, the functional reserve is diminished accordingly (26). Potential advantages quantifying fibrosis with CPA include the a more subjective assessment of liver fibrosis, a broader scale of values allowing better comparison between studies (27), the ability to capture small but potentially important fibrosis changes (especially in the context of therapeutic trials), and to be a better histological reference standard for developing and validating non-invasive fibrosis tests (21,28). Bihari and colleagues recently determined quantitative fibrosis (QF) values for various stages of METAVIR staging in 964 HBV cases. Median QF for F0 was 1% (0.7–1.6%); for F1, 3% (2.1–4.0%); for F2, 7.1% (5.6–8.7%); for F3, 12.7% (10.1–16.7%) and for F4, 26.9% (20.3–36.4%). QF positively correlated with METAVIR staging and hepatic vein pressure gradient along with liver-related outcomes (29).
Despite its continued use, liver biopsy itself is far from an ideal gold standard. The associated high cost, invasiveness, risk of complications, lack of patient acceptance, the need for expert histological interpretation, as well as significant inter-observer and sampling variability limit its use in clinical practice (30). For this reason, current guidelines for management of HBV do not routinely recommend liver biopsy unless non-invasive tests yield indeterminate results (10-12).
Indirect serum markers of fibrosis
Several categories of non-invasive markers utilised for prediction of severity of fibrosis in HBV exist. Of these, indirect markers utilize routine laboratory measures such transaminases, markers of liver synthetic function (albumin, bilirubin, prothrombin time), or other readily available indices which relate to liver disease stage (platelet levels, red cell distribution width). Multiple studies using a combination of these parameters have yielded useful non-invasive scores of fibrosis, and the most commonly used will be discussed here (Table 1) (31-35).
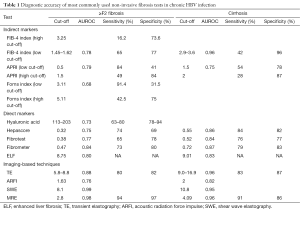
Full table
FIB-4 index
The FIB-4 index was initially developed for chronic hepatitis C virus (HCV)/HIV co-infection and has been subsequently validated for other liver diseases (36,37). The variables entered in the FIB-4 are readily available and include aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet (PLT) count. Using the following formula, age (yr) X AST (U/L)/[(PLT (109/L)]X [ALT (U/L)]1/2, FIB 4 can be calculated (38). The FIB-4 index is an attractive non-invasive fibrosis test as it relies on readily available parameters and is easy to calculate. In a retrospective study by Ma et al. (31) that included 1,168 Chinese HBV patients, the FIB-4 showed a sensitivity of 94%, specificity of 46%, positive predictive value (PPV) of 67%, negative predictive value (NPV) of 87%, and an area under the receiver operator characteristic (AUROC) of 0.79, to distinguish Metavir fibrosis stages F1 and F2 (significant) from F3 and F4 (extensive) at a cut-off value of 1.433–1.858. In a study of 668 Korean HBV patients, Kim et al. (39) also validated the FIB-4 index for prediction of fibrosis in HBV, showing that cut-offs of 1.6 and 3.6 provided an NPV of 93.2% and PPV of 90.8% for ruling in and ruling out cirrhosis respectively. The AUROC values for F2, F3 and F4 fibrosis were 0.865, 0.910 and 0.926 respectively. Mallet et al. (40) showed that the FIB-4 index could classify patients with moderate fibrosis with an AUROC of 0.81 in 138 liver biopsies from French HBV patients. A cut-off value ≤1.45 could differentiate moderate from severe fibrosis with an NPV of 86%, a sensitivity of 71% and a specificity of 73%. A 2014 meta-analysis examined the value of the FIB-4 index for staging fibrosis in approximately 2000 HBV patients. A cut-off of 1.45–1.62 yielded an AUROC value of 0.78 for significant fibrosis, while the AUROC value for cirrhosis was 0.89 at a cut-off of 2.9–3.6 (41). Importantly, the FIB-4 index has also demonstrated value as a prognostic score. In 986 Korean HbsAg carriers, Shuh and colleagues found that a score ≥2.4 gave an adjusted hazard ratio of 21.34 for incidence of HCC compared to subjects with FIB-4 <1.25. Kim et al. noted similar findings in 542 Korean adults with HBV, in whom a FIB-4 cut off of 2.67 showed an AUROC 0.789 for mortality during 5 years of follow-up (42,43). In clinical practice, the low cut-off of FIB-4 at 1.45 can be used to rule out patients without advanced fibrosis, hence it can be used as a triaging test (4). FIB-4 is not reliable in detecting regression of fibrosis following antiviral treatment (44).
APRI
The aspartate aminotransferase (AST)/platelet ratio index (APRI) was proposed by Wai et al. (45) to predict significant fibrosis and cirrhosis in HCV. The formula for calculation of APRI is APRI = [(AST/ULN)/Platelet count] ×100 (ULN = upper limit of normal; 34 U/L for females, 36 U/L for males). The main advantage of APRI over other non-invasive tests is that it is based on readily available blood tests and is simple to use. Although there are many studies evaluating APRI in HBV patients, recent studies have evaluated the role of APRI in HBV. For interpreting APRI, two different scales have previously been proposed. The first scale aims to identify patients with cirrhosis (defined as Ishak stage 5–6); an APRI score >2 is the cut-off used for ruling in whereas a score <1 is used for ruling out cirrhosis respectively. The second scale detects clinically significant fibrosis (Ishak stage 3–6); an APRI score >1.5 is the cut-off for significant fibrosis, whereas a score <0.5 can rule it out (45). In a meta-analysis published in 2012 (46), which included nine studies (n=1,798), the APRI gave AUROCs for significant fibrosis and cirrhosis of 0.79 and 0.75, respectively. For significant fibrosis, an APRI cut-off of 0.5 had a sensitivity of 84% and a specificity of 41%, while a cut-off of 1.5 had a sensitivity of 49% and a specificity of 84%; for cirrhosis a cut-off range of 1.0–1.5 had sensitivity and specificity of 54% and 78% respectively and a cut-off of 2 28% and 87% respectively, leading the authors to conclude that the APRI had limited application in the identification of significant fibrosis or cirrhosis in HBV. A second meta-analysis published in 2014 (47) included 17 studies assessing the APRI for significant fibrosis (3,573 patients) and 11 studies (2,083 patients) examining cirrhosis in HBV. Wide variation in AUROC values were reported, as they ranged from 0.61 to 0.86 (significant fibrosis) and 0.50 to 0.83 (cirrhosis) which was attributed to significant heterogeneity across the studies included. A systematic review and meta-analysis published in 2015 including 16 articles (48), reported that APRI thresholds of 0.5, 1.0, and 1.5 yielded sensitivity and specificity values of 70% and 60%, 50% and 83%, and 36.9% and 92.5% for significant fibrosis, advanced fibrosis, and cirrhosis, respectively. The summary AUROC values using APRI for significant fibrosis, advanced fibrosis, and cirrhosis were 0.74, 0.74, and 0.73, respectively. Like FIB-4, APRI has been used to predict the risk of HCC, HCC recurrence post liver resection and mortality in HBV patients (49-51). A recent study, however, failed to show a correlation between APRI and the regression, stabilisation or progression of fibrosis in 575 HBV patients included in multicentre trials of tenofovir therapy (44). APRI and FIB-4 have been compared with each other in a number of studies. In a recent meta-analysis (52), 71 studies were included and concluded that APRI had lower performances than FIB-4, transient elastography (TE) and FibroTest in both HBV and HCV patients. According to Kim et al., although APRI and FIB-4 scores correlated with Ishak stage at baseline, over 80% of patients with advanced fibrosis or cirrhosis were not picked up by these scores (44). Moreover, neither reductions in APRI nor FIB-4 correlated with fibrosis regression after more than 4 years of antiviral therapy. A meta-analyses evaluating cost-effectiveness of various noninvasive tests to inform treatment decisions in patients with chronic hepatitis B showed that both APRI and FIB4 were not as cost effective as TE (53).
Forns index
Forns et al. (54) constructed a model and a scoring system combining age, GGT, cholesterol, and platelet count that is useful in ruling out patients without significant hepatic fibrosis in HCV. It is calculated as Forns index = 7.811 –3.131 × ln platelet + 0.781 × ln GGT + 3.647 × ln age – 0.014 × cholesterol. When evaluated for HBV, the Forn’s index was modestly useful as a predictor of significant fibrosis (AUROC 0.68) (55,56) and cirrhosis (AUROC 0.7) (56). In a study of 303 Korean patients who had surgical resection for HBV-related HCC, the Forns index also predicted tumour recurrence (Hazard ratio =1.24) and recurrence-free survival mortality (Forns ≥6.9, Hazard ratio =1.2) (57).
AST/ALT ratio (AAR)
The AAR, has been widely utilised as a predictor of cirrhosis in different aetiologies of liver disease. In a study published by Williams et al. in 1988 (58), among 100 patients with HBV, the mean AST/ALT ratio was 0.59 in those without and 1.02 in those with cirrhosis respectively. However, Eminler and colleagues (59) found that the AAR performed inferiorly to other blood-based non-invasive algorithms in estimating the fibrosis stage in 237 HBV patients. Similarly, the ability of the AAR to diagnose significant fibrosis (F2-F4) was poor in a US cohort of 319 HBV patients (AUROC of 0.56) (60).
Other tests
In a study by Poynard et al. (61) of 500 HCV patients, platelet count and age were independently associated with fibrosis. A simple score combining age and platelet count, AST-Platelet Index (API), was created, ranging from 0–10. This index has been applied in HBV with varied results. Kim et al. from Korea (62) found that API was an accurate indicator of cirrhosis (AUROC 0.89) in 346 treatment naïve HBV patients, while a recently published study by Erdogan et al. from Turkey (33) deemed the API as inadequate for evaluation of significant fibrosis in patients with chronic hepatitis B, with an AUROC value of 0.53.
Another index utilises the fact that spleen size increases with advanced fibrosis and portal hypertension to increase the accuracy of API in predicting fibrosis [Age-spleen-platelet ratio index (ASPRI)]. In a Korean study (62), ASPRI showed a high diagnostic accuracy for cirrhosis with an AUROC of 0.893. Using cut-off scores of >12 and <5, the presence or absence of cirrhosis could be correctly identified in 96.3% and 100% of cases, respectively.
The AST/platelet/GGT/AFP (APGA) index is calculated using log index =1.44+0.1490log[GGT (U/L)]+0.3308log[AST (U/L)]–0.5846log[platelet count (×109/L)]+0.1148log[AFP (ng/mL)+1]. In a study by Ozyalvacli et al. (63), the APGA index gave an AUROC of 0.76 for significant fibrosis in 237 treatment naïve HBV patients, while Erdogan et al. (33) found that the AGPA did not fare well for significant fibrosis prediction in a similar cohort of 221 HBV patients, with an AUROC of 0.638.
Seto et al. (64) further developed a novel index (Platelet/Age/Phosphatase/AFP/AST (PAPAS) index) for determining significant fibrosis in HBV. The PAPAS index predicted significant fibrosis with AUROC curve of 0.78. Using the PAPAS index, the authors reported that 67.5% of liver biopsies for patients with ALT<2× ULN would be avoided. However, other studies have since refuted the utility of this index (33,56).
The alpha-fetoprotein (AFP)/activated partial thromboplastin time (APTT)-AA Index was developed in a Chinese cohort of 506 HBV patients, split randomly into estimation and validation cohorts. The AA index is calculated as log index = −9.164 + 0.114 × AFP + 0.236 × APTT. At a low cut-off of 0.007, sensitivity, specificity, PPV, NPV, positive likelihood ratio (LR+) and negative likelihood ratio (LR−) were 91.3, 50, 28.8, 96.3, 1.83 and 0.7, respectively, and at a high cut-off of 0.127, the sensitivity, specificity, PPV, NPV, LR+ and LR– were 65.2, 90, 60, 92.1, 6.52 and 0.39 respectively for the estimation of significant fibrosis, with an AUROC value of 0.82 (56,65).
The Göteborg University Cirrhosis Index (GUCI) may be calculated as = normalised AST × prothrombin-INR ×100/Platelet count (×109/L). It was devised for determining fibrosis in HCV by Islam et al. (65). In the study by Feng et al. (56), an AUROC of 0.72 for significant fibrosis for the GUCI test was found.
The Cirrhosis Discriminate Score (CDS, possible total score 0-11) consists of three laboratory parameters: platelets, ALT/AST ratio, and PT and is calculated as Platelets + ALT/AST + INR (Platelet count (× 109/L): >340=0; 280–339=1; 220–279=2; 16–219=3; 100–159=4; 40–99=5; <40=6, ALT/AST ratio: >1.7=0; 1.2–1.7=1; 0.6–1.19=2; <0.6=3, INR: <1.1=0; 1.1–1.4=1; >1.4=2). Scores >7 showed high specificity (98%) for advanced fibrosis in HCV (66). The test has been examined in 177 HBV patients by Lee et al., who found that a CDS >4 had an 88% specificity and 74% PPV for liver fibrosis (AUROC 0.68) (67).
The GP model (including globulin and platelets) is a model for predicting significant liver fibrosis in HBV patients. It is calculated as Globulin (mg/dL) × 100/PLT (×109/L). Liu et al. (68) derived and validated the GP model in 114 HBV patients, which was further validated in 228 Turkish HBV patients. Using a cut-off of 1.5 yielded an AUROC of 0.74, and a sensitivity, specificity, PPV and NPV of 75.2, 62.8, 62 and 75 respectively for the prediction of significant fibrosis (69).
The Fibrosis Index was developed for prediction of fibrosis in HCV (70). The index is calculated as FI = 8.0 – 0.01 × PLT (109/l) – serum albumin (g/dL), with an F-Index <2.1 indicating no or minimal fibrosis; F-index ≥2.1, significant fibrosis, and if F-Index ≥3.3, significant cirrhosis. At a cut-off of <2, the AUROC, sensitivity, specificity, PPV and NPV were shown to be 0.72, 52.7, 83.2, 72.1 and 68.1 respectively for significant fibrosis in 228 HBV patients (69).
In a retrospective Chinese study of 519 patients (71), red cell distribution width (RDW) was found to increase with progressive liver disease and inflammation. The authors formulated a score called the RDW to platelet ratio (RPR). RPR is calculated as RDW (%)/Platelets (109/L). RPR at a cut-off of 0.077 yielded a good specificity (73.1%) and a modest ability to detect advanced fibrosis (AUC =0.7).
The Wang I and Wang II models were constructed using readily available laboratory parameters (72). Wang I Model is calculated as 10 × eA/(1 + eA) where A = 0.153 – 0.015 × PLT + 0.154 × AST + 0.071 × GGT – 0.226 × ln (HBVDNA). Wang I model cut-off values ≤1.75 and >5.84 were used to identify patients in the immune tolerant phase with or without significant fibrosis, with an AUROC value of 0.87. Wang II is calculated as: Wang II = 10 × eC/(1 + eC), where C = 13.657 – 1.475 × RBC – 0.011 × PLT – 0.019 × TBIL + 0.021 × GGT – 0.052 × PTA – 0.258 × ln (HBVDNA) + 0.160 × BMI. Wang II model cut-off values ≤3.79 and >7.06 were used to rule out and select immune reactive HBeAg-positive patients with or without significant fibrosis respectively, with an AUROC of 0.87.
In a retrospective study of 235 CHB patients (73), body mass index (BMI), platelet count, serum albumin, and total bilirubin levels were independent predictors of bridging fibrosis/cirrhosis. The AUROC of the best model (Hui Model) was comparable in the training cohort and validation cohort (0.80 and 0.76 respectively), with an NPV of 93% in a total of 235 treatment naïve HBV patients.
Alpha2-macroglobulin, age, gamma glutamyl transpeptidase, and hyaluronic acid were used for the Zeng Index (Shanghai Liver Group Model). Using a cutoff score of <3.0 ruled out fibrosis while a score of >8.7 predicted significant fibrosis with high accuracy (74).
The S index consisting of gamma-glutamyltransferase (GGT), platelets (PLT) and albumin (ALB) [S-index: 1,000 × GGT/(PLT × ALB(2)] had an AUROC of 0.81 and 0.89 for predicting significant fibrosis and cirrhosis respectively in 146 HBV patients (75).
Liver stiffness values determined by TE were combined with serum haptoglobin, apolipoprotein A1, and α2-macroglobulin levels to construct a novel model called the HALF index in 208 Korean HBV patients. The AUROC for significant fibrosis was 0.91, with the study authors concluding that 47% of patients could avoid biopsy with an accuracy of 99% (76).
Finally, the Lok score, calculated as: Log odds = –5.56 – 0.0089 × platelet count (103/mm3) + 1.26 × (AST/ALT) + 5.27 × INR Lok = [exp (log odds)]/[1 + EXP (log odds)], was originally developed in large cohort of HCV trial patients (77). When studied in 1,168 Chinese HBV patients, Lok’s model outperformed the APRI and API scores, but not the FIB-4, in the determination of advanced fibrosis (AUROC of 0.71) (31).
The above scores have not been validated in independent datasets, have modest diagnostic accuracy and are not used in routine clinical practice.
Direct serum markers of fibrosis
Hepatic fibrosis is a dynamic process, associated with a cycle of extracellular matrix (ECM) deposition and degradation. Biomarkers that mirror the ECM turnover can be used to assess dynamic changes in liver fibrogenesis (78), therefore both for staging fibrosis but also theoretically to monitor progression or regression. These markers include several glycoproteins, members of the collagen family, collagenases and their inhibitors, and a number of cytokines involved in the fibrogenic process (78). These have been studied individually as well as in panel combinations.
Hyaluronic acid (HA) is the most studied direct serum marker. It is a glycosaminoglycan that is synthesised and distributed throughout the extracellular space by hepatic stellate cells (HSCs) and is degraded by sinusoidal endothelial cells (79). Geramizadeh and colleagues (80), showed significantly higher HA levels in advanced vs. mild-moderate fibrosis in 93 HBV patients. Gümüşay et al. (81) demonstrated the high diagnostic accuracy of HA for predicting ≥F3 fibrosis (AUROC 0.90) in 58 HBV patients.
During the processing of type III procollagen, the PIIINP molecule is produced by a specific N-proteinase. Liver fibrosis alters the metabolism of type III collagen resulting in changes in serum PIIINP concentration, with the potential clinical application. In 200 Chinese HBV patients, Chang et al. (82) found serum PIIIP levels significantly elevated in acute hepatitis, chronic persistent hepatitis, and inactive cirrhosis. A later study found out that 56% of HBV patients had normal serum PIIINP levels at presentation, limiting its use as a diagnostic marker of liver injury or as a tool for monitoring the response to interferon (83).
Laminin (LN) is a non-collagenous glycoprotein synthesised by HSCs, which is deposited in the basement membrane of the liver. During fibrosis, laminin accumulates around the vessels, in the perisinusoidal spaces, and near the portal tract (84). Li et al. (85) found that serum LN levels increased significantly with increasing liver fibrosis in 87 Chinese HBV patients. At a cut-off value of serum LN 132.7 ng/mL, the sensitivity, specificity, PPV, NPV, LR+, LR– and AC were 72%, 80%, 87%, 60%, 3.6%, 0.35% and 74.7%, respectively for significant fibrosis.
The imbalance between MMPs and TIMPs is thought to be an important determinant of ECM deposition and breakdown. TIMP-1 has been particularly studied as a candidate biomarker of liver fibrosis. In 159 Chinese HBV patients, Zhu et al. (86) found significant associations between serum TIMP-1 levels, hepatic inflammation and fibrosis grades. The AUROC of serum TIMP-1 was 0.92 for significant liver fibrosis (≥stage 2), with sensitivity 89.4% and specificity 83.6% at a cut-off value ≥174.5 ng/mL. Seven et al. (87) concluded that TIMP-1 and HA were powerful predictors of fibrosis (odds ratio’s of 8.65 and 8.38) in 109 patients with HBV and hepatitis D (HDV) infection. Another small study found that TIMP-1 mRNA levels in combination with platelet-derived growth factor-BB (PDGF-BB) gave sensitivity and specificity values for liver fibrosis of 97.4% and 95%, respectively (88).
Direct markers have been used in combined panels to increase the diagnostic performance of a single parameter Fibrometer® is a patented test combining age, platelets, HA, AST, prothrombin index, urea, and α2-macroglobulin. In 78 HBV patients, Wu and colleagues found that the Fibrometer test performed well in 78 HBV patients, diagnosing significant and severe fibrosis with AUROC values of 0.85 and 0.94, respectively (89). Another study showed that although Fibrometer showed good diagnostic accuracy in HBV, it tended to underestimate significant fibrosis when compared with HCV (90).
Hepascore® is a patented test that consists of age, gender, HA, bilirubin, gamma-glutamyl-transpeptidase (γGT), and α2-marcoglobulin. Hepascore® is an automated panel test that requires a single analyser and serum sample. A recent meta-analysis of the use of Hepascore in chronic liver disease included 21 studies, with 588 HBV patients (91). Combining HBV studies, the mean adjusted AUROC was 0.83 for significant fibrosis, 0.91 for advanced fibrosis and 0.92 for cirrhosis
The enhanced liver fibrosis (ELF) panel combines HA, TIMP-1 and PIIINP. In a study of 182 HBV patients (92), using ELF to identify severe fibrosis at cut-offs of 9.08 and 9.94, 60% of patients would have correctly avoided liver biopsy, and 16% incorrectly. The AUROC values for any fibrosis and cirrhosis were 0.77 and 0.83, respectively. A study published the same year from Asia in 170 HBV patients showed that the ELF test had an AUROC of 0.81 to predict liver-related events, higher than liver stiffness by TE and histological fibrosis grade (93). Trembling et al. concluded in their study that although ELF has good performance in detection of liver fibrosis in patients with CHB, TE performs better in identifying severe fibrosis/cirrhosis (92).
The FibroTest/FibroSure (FT) is a patented test that combines five serum biochemical parameters (α-2-macroglobulin, apolipoprotein A1, haptoglobin, L-glutamyl transpeptidase, and bilirubin) developed by Poynard et al. initially in patients with HCV (94). Its use in HBV was the subject of a recent meta-analysis, which included 16 studies (n=2,494) for fibrosis and 13 studies (n=1,754) for cirrhosis. An FT threshold of 0.48 gave a sensitivity and specificity for significant fibrosis of 61% and 80%, with a summary ROC of 0.84. A threshold of 0.74 gave a sensitivity and specificity for cirrhosis of 62% and 91%, respectively, with a summary ROC of 0.87. The authors concluded that FibroTest is useful in ruling out CHB-related cirrhosis, but has suboptimal accuracy in diagnosing significant fibrosis and cirrhosis (95).
Imaging-based techniques
Liver stiffness measurement: transient elastography (TE)—Fibroscan
Fibroscan (Echosens, Paris, France) utilises the principle of vibration controlled tissue elastography (VCTE), where a vibration pressure wave generated by a probe is detected by the transducer from the same probe when it travels through the liver. The stiffer the liver, the higher is the velocity, indicated by a numeric value between 4.0 to 75 kPa. TE is an easily-performed, rapid bedside test, with immediate read-out for clinical use. TE has been validated for fibrosis assessment in several liver diseases including HBV. In reality, TE does not directly measure liver fibrosis; it is a measure of liver stiffness (LS) that has been associated with the degree of fibrosis.
In a 2015 meta-analysis of 27 studies comprising 4,386 HBV patients, TE showed high diagnostic accuracy for the detection of liver fibrosis. For staging fibrosis F≥2, F≥3 and F=4, the summary sensitivity was 0.81, 0.82 and 0.86, respectively, the summary specificity was 0.82, 0.87 and 0.87, respectively, and the corresponding AUROC was 0.88, 0.91 and 0.93, respectively (96). The cut-off values for significant fibrosis (≥F2) ranged from 5.8 to 8.8 kPa, for fibrosis ≥F3 from 7.0 to 13.5 kPa, and for cirrhosis (F4) from 9.0 to 16.9 kPa (97-103). A recent study showed the utility of TE for the diagnosis of fibrosis in 263 HBV patients with ALT levels <2× upper limit of normal (ULN), particularly in those with at least significant underlying fibrosis. LS value was also found to correlate significantly with both liver fibrosis and necroinflammatory activity on biopsy, a consideration for the interpretation of TE measurements (104). Some authors have suggested that TE cut-offs should incorporate ALT levels which fluctuate with inflammation in HBV, and TE may be particularly useful HbeAg-negative patients with normal LFTs to guide the need for biopsy of treatment (105). TE has also shown value as a predictor of liver-related outcomes in HBV. For instance, Kim et al. found good diagnostic accuracy to predict HCC in 1308 HBV patients, with AUROC values predicting HCC risk at 3, 5 and 7 years of 0.79–0.81, which was superior to FIB-4 (7). Another study of 381 HBV patients starting therapy found increasing cumulative incidence rates of HCC in association with elevated LS values in 3 stratified groups (LS <8, 8–13, and >13 kPa), and while LS was an independent predictor of HCC development, histological staging was not (106).
There are limitations associated with transient elastography, including the confounding effects of inflammatory activity and steatosis of liver stiffness values (107). TE requires a dedicated machine and operator training, and the costs associated. TE has not been as extensively validated for HBV as In HCV, with typically lower cut-off values for fibrosis detection in HBV noted (108). While accurate for diagnosing of cirrhosis, TE suffers from reduced accuracy in lower fibrosis stages, similar to blood-based biomarkers, but nevertheless TE represents an invaluable addition to the clinician’s toolkit.
Liver stiffness measurement: acoustic radiation force impulse (ARFI) elastography
ARFI elastography uses radiation-forced impulses to measure LS while using B-mode ultrasonography. In contrast to TE which has a fixed region of interest (ROI) at a fixed insertion depth, ARFI elastography has a flexible ROI at variable depths which enables LS measurement in patients with ascites and obesity. Most studies of ARFI in Europe are for HCV, and for HBV in Asia. In a study of 250 HBV patients (109), ARFI showed comparable efficacy to TE for the detection of significant fibrosis (≥F2) and cirrhosis (F4). ARFI AUROC values for ≥F2 and F4 were 0.74 and 0.79 respectively. The optimum cut-off values for ARFI decreased for both ≥F2 and F4 in a subgroup of 131 patients with normal ALT levels, highlighting the need to interpret LS values alongside biochemistry in HBV patients (109). Park and colleagues recently showed that advanced fibrosis is a predictor of non-discordance between biopsy and ARFI. Most discordances were caused by the overestimation of liver fibrosis by ARFI in patients with F0–2, as well as high BMI (110). As seen on TE, LS measurements by ARFI had higher AUROCs for cirrhosis prediction in HCV than HBV (0.824 vs. 0.707), with inflammation impacting on ARFI values in HBV (111). No significant differences in AUROC values for fibrosis stages between ARFI and TE were noted, with reported AUROCs of 0.76 and 0.81 (≥stage 2), 0.85 and 0.85 (≥stage 3) and 0.82 and 0.80 (S =4), respectively. Similar to other studies, optimum cut-off values decreased in patients with normal ALT levels (112).
Liver fibrosis index (LFI): real-time shear wave elastography (SWE)
Shear Wave Elastography is a novel real-time two-dimensional (2D) elastography technique, which allows one to estimate stiffness quantitatively in kilopascals (kPa). Moreover, overlapping elastography over regular B-mode allows precise choice of the region of interest, unlike TE. 2D-SWE was compared to TE in 226 HBV patients and 171 healthy controls, with the highest AUROC values achieved by 2D-SWE across all fibrosis grades (AUROC 0.86 for fibrosis (≥ F1 stage); 0.88 for moderate fibrosis (≥ F2 stage); 0.93 for severe fibrosis (≥ F3 stage); and 0.98 for cirrhosis. In this study, 2D-SWE also had higher successful acquisition rate than TE (98.9% vs. 89.6%) (113). In a study of 123 HCV and HBV patients, 2D-SWE accurately differentiated fibrosis stages, with cut-off values of 8.1 (AUC =0.99) for F ≥ 3, 10.8 kPa (AUC =0.95) for cirrhosis, and 27 kPa (AUC =0.96) for decompensated cirrhosis (114). 2D-SWE was further shown to better discriminate between ≥F3 and F4 thanFIB-4 and APRI scores, but like other tests, in HCV patients, the AUROC value of 2D-SWE for advanced fibrosis is higher than that in HCV patients (115).
MR elastography
Magnetic Resonance Elastography, a modified contrast technique developed to characterise the elasticity of the tissues, is a new technique employed for non-invasive assessment of liver fibrosis. It is a non-invasive, reproducible, advanced diagnostic technique for staging hepatic fibrosis (116). Wu and colleagues found that MRE predicted fibrosis stages better than APRI in 160 patients with chronic viral hepatitis and 25 healthy controls. An MRE cutoff value of 2.80 kPa, gave a sensitivity of 94.4% and a specificity of 97.8% for detecting significant fibrosis (≥F2) (117). In a study of 195 HBV patients and 166 living donor candidates, The cut-off values of MRE LS for ≥F1, ≥F2, ≥F3, and F4 were 2.45, 2.69, 3.0, and 3.94 kPa, respectively, yielding AUROC values of 0.99. 50% of patients assessed in this study had F0 stage. In contrast to TE, MRE did not correlate with necroinflammatory on biopsy, and the technical success rate was 92.5% (116). While the diagnostic performance of MRE in HBV patients appears similar to that in HCV (117,118) necroinflammation may contribute to increased hepatic stiffness by MRE in HBV patients with ≤F2 fibrosis (119). MRE using three-dimensional spin-echo echo planar imaging (3D-SE-EPI) is a novel approach associated with a 2.2% failure rate and high diagnostic accuracy (AUROC values for ≥F1, ≥F2, ≥F3, and F4 of 0.957 to 0.991) in 179 patients with HBV or HCV (120). Other MR-based imaging techniques to assess fibrosis including diffusion weighted imaging, dynamic contrast enhanced MRI and multi-parametric MRI are currently in development and await further validation (121).
Combination of tests
In order to increase the diagnostic accuracy of non-invasive tests, combined models utilising two or more tests have been applied. Li et al. used a dual approach to stage 307 HBV patients, combining APRI or FIB-4 with LS by Fibroscan, resulting in a significant reduction in the need for liver biopsy compared to either test alone. Less than 4% patients required a biopsy to confirm cirrhosis following screening with combined tests (103) Liang et al. also explored a stepwise application of TE with APRI or FIB-4 in 236 HBV patients with ALT levels <5× ULN, and found an increase in PPV for cirrhosis from 0.677 to 0.808 and 0.724, respectively. They also found that a remarkable 76% of biopsies to confirm cirrhosis were avoided with this approach (122). Recently, Lee and colleagues proposed a novel combination model in a training and validation cohort of 492 HBV patients called the LAW (liver stiffness, APRI, woman) index. Calculated as: 1.5 × liver stiffness (kPa) + 3.9 × APRI + 3.2 if female, the LAW index was a better predictor of A3F2 [necroinflammatory grade ≥A3 or fibrosis grade ≥F2 (A3F2)] than the APRI or LS by TE alone in both training group (AUROC 0.862–0.870) (123).
Conclusions
In summary, a battery of non-invasive markers is available for the determination of fibrosis and monitoring the progression and regression of fibrosis in chronic HBV patients. The selection of the tests depends on individual patient factors, as well as the cost, accuracy, reliability and availability of these tests. With the constant evolution of non-invasive tests for fibrosis, which demonstrate excellent diagnostic performance for cirrhosis, as well as prognostic prediction of liver-related outcomes, the role of liver biopsy is becoming less prominent. Specifically for chronic HBV infection, treatment decisions sometimes depend on the presence of necroinflammation rather than fibrosis, therefore in such cases liver histology is still irreplaceable. The challenge now is to decide on how best to apply validated non-invasive tests in HBV management. It is likely that a combination approach (i.e., blood and imaging test at screening) will give the highest diagnostic accuracy, obviate the need for the greatest number of liver biopsies, and inform the clinician and patient regarding prognosis and the need for therapy. Furthermore, a consensus on the use of non-invasive markers to replace liver biopsy as trial endpoints would greatly enhance HBV clinical research trials, ultimately benefiting the patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hepatitis B. 2002(WHO/CDC/CSR/LYO/2002.2:Hepatitis B). Available online: http://apps.who.int/iris/bitstream/10665/67746/1/WHO_CDS_CSR_LYO_2002.2_HEPATITIS_B.pdf
- McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009;49:S45-55. [Crossref] [PubMed]
- Guo GH, Tan DM, Zhu PA, et al. Hepatitis B virus X protein promotes proliferation and upregulates TGF-beta1 and CTGF in human hepatic stellate cell line, LX-2. Hepatobiliary Pancreat Dis Int 2009;8:59-64. [PubMed]
- Crossan C, Tsochatzis EA, Longworth L, et al. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: systematic review and economic evaluation. Health Technol Assess 2015;19:1-409. v-vi. [Crossref] [PubMed]
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749-61. [Crossref] [PubMed]
- Weissberg JI, Andres LL, Smith CI, et al. Survival in chronic hepatitis B. An analysis of 379 patients. Ann Intern Med 1984;101:613-6. [Crossref] [PubMed]
- Kim SU, Kim BK, Park JY, et al. Transient Elastography is Superior to FIB-4 in Assessing the Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. Medicine (Baltimore) 2016;95:e3434. [Crossref] [PubMed]
- Ijaz B, Ahmad W, Javed FT, et al. Revised cutoff values of ALT and HBV DNA level can better differentiate HBeAg (-) chronic inactive HBV patients from active carriers. Virol J 2011;8:86. [Crossref] [PubMed]
- Bárcena Marugán R, García Garzón S. DNA-guided hepatitis B treatment, viral load is essential, but not sufficient. World J Gastroenterol 2009;15:423-30. [Crossref] [PubMed]
- Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98. [Crossref] [PubMed]
- Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261-83. [Crossref] [PubMed]
- European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-85. [Crossref] [PubMed]
- Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013;381:468-75. [Crossref] [PubMed]
- Papachrysos N, Hytiroglou P, Papalavrentios L, et al. Antiviral therapy leads to histological improvement of HBeAg-negative chronic hepatitis B patients. Ann Gastroenterol 2015;28:374-8. [PubMed]
- European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237-64. [Crossref] [PubMed]
- Papastergiou V, Tsochatzis E, Burroughs AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol 2012;25:218-31. [PubMed]
- Mani H, Kleiner DE. Liver biopsy findings in chronic hepatitis B. Hepatology 2009;49:S61-71. [Crossref] [PubMed]
- Fernández-Rodríguez CM, Gutiérrez-García ML. Prevention of hepatocellular carcinoma in patients with chronic hepatitis B. World J Gastrointest Pharmacol Ther 2014;5:175-82. [Crossref] [PubMed]
- Grizzi F, Desmet VJ. Liver biopsy interpretation & the regression of hepatitis B virus related cirrhosis. Indian J Med Res 2014;140:160-2. [PubMed]
- Germani G, Burroughs AK, Dhillon AP. The relationship between liver disease stage and liver fibrosis: a tangled web. Histopathology 2010;57:773-84. [Crossref] [PubMed]
- Yegin EG, Yegin K, Ozdogan OC. Digital image analysis in liver fibrosis: basic requirements and clinical implementation. Biotechnology & Biotechnological Equipment. 2016;30:653-60. [Crossref]
- Calvaruso V, Dhillon AP, Tsochatzis E, et al. Liver collagen proportionate area predicts decompensation in patients with recurrent hepatitis C virus cirrhosis after liver transplantation. J Gastroenterol Hepatol 2012;27:1227-32. [Crossref] [PubMed]
- Tsochatzis E, Bruno S, Isgro G, et al. Collagen proportionate area is superior to other histological methods for sub-classifying cirrhosis and determining prognosis. J Hepatol 2014;60:948-54. [Crossref] [PubMed]
- Manousou P, Dhillon AP, Isgro G, et al. Digital image analysis of liver collagen predicts clinical outcome of recurrent hepatitis C virus 1 year after liver transplantation. Liver Transpl 2011;17:178-88. [Crossref] [PubMed]
- Manousou P, Burroughs AK, Tsochatzis E, et al. Digital image analysis of collagen assessment of progression of fibrosis in recurrent HCV after liver transplantation. J Hepatol 2013;58:962-8. [Crossref] [PubMed]
- Xie SB, Ma C, Lin CS, et al. Collagen proportionate area of liver tissue determined by digital image analysis in patients with HBV-related decompensated cirrhosis. Hepatobiliary Pancreat Dis Int 2011;10:497-501. [Crossref] [PubMed]
- Tsochatzis EA, Manousou P, Fede G, et al. Validating non-invasive markers of fibrosis: the need for a new histological reference standard. Gut 2011;60:1442-3; author reply 1443-4. [Crossref] [PubMed]
- Tsochatzis EA, Germani G, Hall A, et al. Noninvasive assessment of liver fibrosis: the need for better validation. Hepatology 2011;53:1781-2; author reply 1782-3.
- Bihari C, Rastogi A, Sen B, et al. Quantitative fibrosis estimation by image analysis predicts development of decompensation, composite events and defines event-free survival in chronic hepatitis B patients. Hum Pathol 2016;55:63-71. [Crossref] [PubMed]
- ter Borg F, ten Kate FJ, Cuypers HT, et al. A survey of liver pathology in needle biopsies from HBsAg and anti-HBe positive individuals. J Clin Pathol 2000;53:541-8. [Crossref] [PubMed]
- Ma J, Jiang Y, Gong G. Evaluation of seven noninvasive models in staging liver fibrosis in patients with chronic hepatitis B virus infection. Eur J Gastroenterol Hepatol 2013;25:428-34. [Crossref] [PubMed]
- Chen B, Ye B, Zhang J, et al. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS One 2013;8:e68780. [Crossref] [PubMed]
- Erdogan S, Dogan HO, Sezer S, et al. The diagnostic value of non-invasive tests for the evaluation of liver fibrosis in chronic hepatitis B patients. Scand J Clin Lab Invest 2013;73:300-8. [Crossref] [PubMed]
- Lombardi R, Buzzetti E, Roccarina D, et al. Non-invasive assessment of liver fibrosis in patients with alcoholic liver disease. World J Gastroenterol 2015;21:11044-52. [Crossref] [PubMed]
- Buzzetti E, Lombardi R, De Luca L, et al. Noninvasive Assessment of Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Int J Endocrinol 2015;2015:343828. [Crossref] [PubMed]
- McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265-9. [Crossref] [PubMed]
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317-25. [Crossref] [PubMed]
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32-6. [Crossref] [PubMed]
- Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546-53. [Crossref] [PubMed]
- Mallet V, Dhalluin-Venier V, Roussin C, et al. The accuracy of the FIB-4 index for the diagnosis of mild fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2009;29:409-15. [Crossref] [PubMed]
- Li Y, Chen Y, Zhao Y. The diagnostic value of the FIB-4 index for staging hepatitis B-related fibrosis: a meta-analysis. PLoS One 2014;9:e105728. [Crossref] [PubMed]
- Suh B, Park S, Shin DW, et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology 2015;61:1261-8. [Crossref] [PubMed]
- Kim JH, Kim JW, Seo JW, et al. Noninvasive Tests for Fibrosis Predict 5-Year Mortality and Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. J Clin Gastroenterol 2016;50:882-8. [Crossref] [PubMed]
- Kim WR, Berg T, Asselah T, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol 2016;64:773-80. [Crossref] [PubMed]
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518-26. [Crossref] [PubMed]
- Jin W, Lin Z, Xin Y, et al. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC Gastroenterol 2012;12:14. [Crossref] [PubMed]
- Xu XY, Kong H, Song RX, et al. The effectiveness of noninvasive biomarkers to predict hepatitis B-related significant fibrosis and cirrhosis: a systematic review and meta-analysis of diagnostic test accuracy. PLoS One 2014;9:e100182. [Crossref] [PubMed]
- Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 2015;61:292-302. [Crossref] [PubMed]
- Mao W, Sun Q, Fan J, et al. AST to Platelet Ratio Index Predicts Mortality in Hospitalized Patients With Hepatitis B-Related Decompensated Cirrhosis. Medicine (Baltimore) 2016;95:e2946. [Crossref] [PubMed]
- Hann HW, Wan S, Lai Y, et al. Aspartate aminotransferase to platelet ratio index as a prospective predictor of hepatocellular carcinoma risk in patients with chronic hepatitis B virus infection. J Gastroenterol Hepatol 2015;30:131-8. [Crossref] [PubMed]
- Shen SL, Fu SJ, Chen B, et al. Preoperative aspartate aminotransferase to platelet ratio is an Independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol 2014;21:3802-9. [Crossref] [PubMed]
- Houot M, Ngo Y, Munteanu M, et al. Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Aliment Pharmacol Ther 2016;43:16-29. [Crossref] [PubMed]
- Crossan C, Tsochatzis A, Longworth L, et al. Cost-effectiveness of noninvasive liver fibrosis tests for treatment decisions in patients with chronic hepatitis B in the UK: systematic review and economic evaluation. J Viral Hepat 2016;23:139-49. [Crossref] [PubMed]
- Forns X, Ampurdanès S, Llovet JM, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002;36:986-92. [Crossref] [PubMed]
- Ucar F, Sezer S, Ginis Z, et al. APRI, the FIB-4 score, and Forn's index have noninvasive diagnostic value for liver fibrosis in patients with chronic hepatitis B. Eur J Gastroenterol Hepatol 2013;25:1076-81. [Crossref] [PubMed]
- Feng L, Sun K, Zhang J, et al. A novel non-invasive index using AFP and APTT is associated with liver fibrosis in patients with chronic hepatitis B infection: a retrospective cohort study. BMJ Open 2015;5:e008032. [Crossref] [PubMed]
- Choi WM, Lee JH, Ahn H, et al. Forns index predicts recurrence and death in patients with hepatitis B-related hepatocellular carcinoma after curative resection. Liver Int 2015;35:1992-2000. [Crossref] [PubMed]
- Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 1988;95:734-9. [Crossref] [PubMed]
- Eminler AT, Ayyildiz T, Irak K, et al. AST/ALT ratio is not useful in predicting the degree of fibrosis in chronic viral hepatitis patients. Eur J Gastroenterol Hepatol 2015;27:1361-6. [Crossref] [PubMed]
- Teshale E, Lu M, Rupp LB, et al. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS). J Viral Hepat 2014;21:917-20. [Crossref] [PubMed]
- Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepat 1997;4:199-208. [Crossref] [PubMed]
- Kim BK, Kim SA, Park YN, et al. Noninvasive models to predict liver cirrhosis in patients with chronic hepatitis B. Liver Int 2007;27:969-76. [Crossref] [PubMed]
- Ozyalvacli G, Kucukbayrak A, Kurt M, et al. Non-invasive fibrosis tests are correlated with necroinflammatory actvity of liver in patients with chronic hepatitis B. Clin Ter 2014;165:e199-204. [PubMed]
- Seto WK, Lee CF, Lai CL, et al. A new model using routinely available clinical parameters to predict significant liver fibrosis in chronic hepatitis B. PLoS One 2011;6:e23077. [Crossref] [PubMed]
- Islam S, Antonsson L, Westin J, et al. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol 2005;40:867-72. [Crossref] [PubMed]
- Bonacini M, Hadi G, Govindarajan S, et al. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1997;92:1302-4. [PubMed]
- Lee IC, Chan CC, Huang YH, et al. Comparative analysis of noninvasive models to predict early liver fibrosis in hepatitis B e Antigen-negative Chronic Hepatitis B. J Clin Gastroenterol 2011;45:278-85. [Crossref] [PubMed]
- Liu XD, Wu JL, Liang J, et al. Globulin-platelet model predicts minimal fibrosis and cirrhosis in chronic hepatitis B virus infected patients. World J Gastroenterol 2012;18:2784-92. [Crossref] [PubMed]
- Coskun BD, Altinkaya E, Sevinc E, et al. The diagnostic value of a globulin/platelet model for evaluating liver fibrosis in chronic hepatitis B patients. Rev Esp Enferm Dig 2015;107:740-4. [Crossref] [PubMed]
- Ohta T, Sakaguchi K, Fujiwara A, et al. Simple surrogate index of the fibrosis stage in chronic hepatitis C patients using platelet count and serum albumin level. Acta Med Okayama 2006;60:77-84. [PubMed]
- Xu WS, Qiu XM, Ou QS, et al. Red blood cell distribution width levels correlate with liver fibrosis and inflammation: a noninvasive serum marker panel to predict the severity of fibrosis and inflammation in patients with hepatitis B. Medicine (Baltimore) 2015;94:e612. [Crossref] [PubMed]
- Wan R, Liu H, Wang X, et al. Noninvasive predictive models of liver fibrosis in patients with chronic hepatitis B. Int J Clin Exp Med 2015;8:961-71. [PubMed]
- Hui AY, Chan HL, Wong VW, et al. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am J Gastroenterol 2005;100:616-23. [Crossref] [PubMed]
- Zeng MD, Lu LG, Mao YM, et al. Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology 2005;42:1437-45. [Crossref] [PubMed]
- Zhou K, Gao CF, Zhao YP, et al. Simpler score of routine laboratory tests predicts liver fibrosis in patients with chronic hepatitis B. J Gastroenterol Hepatol 2010;25:1569-77. [Crossref] [PubMed]
- Lee HJ, Seo YS, Kim DJ, et al. Application of the HALF index obviates the need for liver biopsy in half of all patients with chronic hepatitis B. J Gastroenterol Hepatol 2011;26:987-95. [Crossref] [PubMed]
- Lok AS, Ghany MG, Goodman ZD, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology 2005;42:282-92. [Crossref] [PubMed]
- Valva P, Ríos DA, De Matteo E, et al. Chronic hepatitis C virus infection: Serum biomarkers in predicting liver damage. World J Gastroenterol 2016;22:1367-81. [Crossref] [PubMed]
- Shiha G. Serum hyaluronic acid: a promising marker of hepatic fibrosis in chronic hepatitis B. Saudi J Gastroenterol 2008;14:161-2. [Crossref] [PubMed]
- Geramizadeh B, Janfeshan K, Saberfiroozi M. Serum hyaluronic acid as a noninvasive marker of hepatic fibrosis in chronic hepatitis B. Saudi J Gastroenterol 2008;14:174-7. [Crossref] [PubMed]
- Gümüşay O, Ozenirler S, Atak A, et al. Diagnostic potential of serum direct markers and non-invasive fibrosis models in patients with chronic hepatitis B. Hepatol Res 2013;43:228-37. [Crossref] [PubMed]
- Chang TT, Lin HC, Lee SD, et al. Clinical significance of serum type-III procollagen aminopropeptide in hepatitis B virus-related liver diseases. Scand J Gastroenterol 1989;24:533-8. [Crossref] [PubMed]
- Giustina G, Fattovich G, De Paoli M, et al. Serum procollagen type III peptide in chronic hepatitis B. Relationship to disease activity and response to interferon-alpha therapy. Int J Clin Lab Res 1996;26:33-6. [Crossref] [PubMed]
- Kropf J, Gressner AM, Negwer A. Efficacy of serum laminin measurement for diagnosis of fibrotic liver diseases. Clin Chem 1988;34:2026-30. [PubMed]
- Li F, Zhu CL, Zhang H, et al. Role of hyaluronic acid and laminin as serum markers for predicting significant fibrosis in patients with chronic hepatitis B. Braz J Infect Dis 2012;16:9-14. [Crossref] [PubMed]
- Zhu CL, Li WT, Li Y, et al. Serum levels of tissue inhibitor of metalloproteinase-1 are correlated with liver fibrosis in patients with chronic hepatitis B. J Dig Dis 2012;13:558-63. [Crossref] [PubMed]
- Seven G, Karatayli SC, Köse SK, et al. Serum connective tissue markers as predictors of advanced fibrosis in patients with chronic hepatitis B and D. Turk J Gastroenterol 2011;22:305-14. [Crossref] [PubMed]
- Cai WM, Zhang BB, Weng HL, et al. The diagnostic value of eight serum indices for liver fibrosis. Zhonghua Gan Zang Bing Za Zhi 2004;12:219-22. [PubMed]
- Wu SD, Wang JY, Li L. Staging of liver fibrosis in chronic hepatitis B patients with a composite predictive model: a comparative study. World J Gastroenterol 2010;16:501-7. [Crossref] [PubMed]
- Leroy V, Sturm N, Faure P, et al. Prospective evaluation of FibroTest®, FibroMeter®, and HepaScore® for staging liver fibrosis in chronic hepatitis B: comparison with hepatitis C. J Hepatol 2014;61:28-34. [Crossref] [PubMed]
- Huang Y, Adams LA, Joseph J, et al. The ability of Hepascore to predict liver fibrosis in chronic liver disease: a meta-analysis. Liver Int 2017;37:121-31. [Crossref] [PubMed]
- Trembling PM, Lampertico P, Parkes J, et al. Performance of Enhanced Liver Fibrosis test and comparison with transient elastography in the identification of liver fibrosis in patients with chronic hepatitis B infection. J Viral Hepat 2014;21:430-8. [Crossref] [PubMed]
- Kim BK, Kim HS, Yoo EJ, et al. Risk assessment of clinical outcomes in Asian patients with chronic hepatitis B using enhanced liver fibrosis test. Hepatology 2014;60:1911-9. [Crossref] [PubMed]
- Imbert-Bismut F, Ratziu V, Pieroni L, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001;357:1069-75. [Crossref] [PubMed]
- Salkic NN, Jovanovic P, Hauser G, et al. FibroTest/Fibrosure for significant liver fibrosis and cirrhosis in chronic hepatitis B: a meta-analysis. Am J Gastroenterol 2014;109:796-809. [Crossref] [PubMed]
- Li Y, Huang YS, Wang ZZ, et al. Systematic review with meta-analysis: the diagnostic accuracy of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B. Aliment Pharmacol Ther 2016;43:458-69. [Crossref] [PubMed]
- Wong GL, Wong VW, Choi PC, et al. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2010;31:1095-103. [Crossref] [PubMed]
- Degos F, Perez P, Roche B, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 2010;53:1013-21. [Crossref] [PubMed]
- Oliveri F, Coco B, Ciccorossi P, et al. Liver stiffness in the hepatitis B virus carrier: a non-invasive marker of liver disease influenced by the pattern of transaminases. World J Gastroenterol 2008;14:6154-62. [Crossref] [PubMed]
- Chan HL, Wong GL, Choi PC, et al. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat 2009;16:36-44. [Crossref] [PubMed]
- Marcellin P, Ziol M, Bedossa P, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int 2009;29:242-7. [Crossref] [PubMed]
- Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61-9. [Crossref] [PubMed]
- Li Y, Cai Q, Zhang Y, et al. Development of algorithms based on serum markers and transient elastography for detecting significant fibrosis and cirrhosis in chronic hepatitis B patients: Significant reduction in liver biopsy. Hepatol Res 2016;46:1367-79. [Crossref] [PubMed]
- Huang R, Jiang N, Yang R, et al. Fibroscan improves the diagnosis sensitivity of liver fibrosis in patients with chronic hepatitis B. Exp Ther Med 2016;11:1673-7. [PubMed]
- Castera L. Hepatitis B: are non-invasive markers of liver fibrosis reliable? Liver Int 2014;34 Suppl 1:91-6. [Crossref] [PubMed]
- Seo YS, Kim MN, Kim SU, et al. Risk Assessment of Hepatocellular Carcinoma Using Transient Elastography Vs. Liver Biopsy in Chronic Hepatitis B Patients Receiving Antiviral Therapy. Medicine (Baltimore) 2016;95:e2985. [Crossref] [PubMed]
- Wong GL, Wong VW, Chim AM, et al. Factors associated with unreliable liver stiffness measurement and its failure with transient elastography in the Chinese population. J Gastroenterol Hepatol 2011;26:300-5. [Crossref] [PubMed]
- Tsochatzis EA, Gurusamy KS, Ntaoula S, et al. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 2011;54:650-9. [Crossref] [PubMed]
- Yoon KT, Lim SM, Park JY, et al. Liver stiffness measurement using acoustic radiation force impulse (ARFI) elastography and effect of necroinflammation. Dig Dis Sci 2012;57:1682-91. [Crossref] [PubMed]
- Park MS, Kim SW, Yoon KT, et al. Factors Influencing the Diagnostic Accuracy of Acoustic Radiation Force Impulse Elastography in Patients with Chronic Hepatitis B. Gut Liver 2016;10:275-82. [Crossref] [PubMed]
- Tai DI, Tsay PK, Jeng WJ, et al. Differences in liver fibrosis between patients with chronic hepatitis B and C: evaluation by acoustic radiation force impulse measurements at 2 locations. J Ultrasound Med 2015;34:813-21. [Crossref] [PubMed]
- Zhang D, Chen M, Wang R, et al. Comparison of acoustic radiation force impulse imaging and transient elastography for non-invasive assessment of liver fibrosis in patients with chronic hepatitis B. Ultrasound Med Biol 2015;41:7-14. [Crossref] [PubMed]
- Leung VY, Shen J, Wong VW, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology 2013;269:910-8. [Crossref] [PubMed]
- Grgurevic I, Puljiz Z, Brnic D, et al. Liver and spleen stiffness and their ratio assessed by real-time two dimensional-shear wave elastography in patients with liver fibrosis and cirrhosis due to chronic viral hepatitis. Eur Radiol 2015;25:3214-21. [Crossref] [PubMed]
- Kim YW, Kwon JH, Jang JW, et al. Diagnostic usefulness of real-time elastography for liver fibrosis in chronic viral hepatitis B and C. Gastroenterol Res Pract 2014;2014:210407.
- Lee JE, Lee JM, Lee KB, et al. Noninvasive assessment of hepatic fibrosis in patients with chronic hepatitis B viral infection using magnetic resonance elastography. Korean J Radiol 2014;15:210-7. [Crossref] [PubMed]
- Wu WP, Chou CT, Chen RC, et al. Non-Invasive Evaluation of Hepatic Fibrosis: The Diagnostic Performance of Magnetic Resonance Elastography in Patients with Viral Hepatitis B or C. PLoS One 2015;10:e0140068. [Crossref] [PubMed]
- Chang W, Lee JM, Yoon JH, et al. Liver Fibrosis Staging with MR Elastography: Comparison of Diagnostic Performance between Patients with Chronic Hepatitis B and Those with Other Etiologic Causes. Radiology 2016;280:88-97. [Crossref] [PubMed]
- Shi Y, Guo Q, Xia F, et al. MR elastography for the assessment of hepatic fibrosis in patients with chronic hepatitis B infection: does histologic necroinflammation influence the measurement of hepatic stiffness? Radiology 2014;273:88-98. [Crossref] [PubMed]
- Shi Y, Xia F, Li QJ, et al. Magnetic Resonance Elastography for the Evaluation of Liver Fibrosis in Chronic Hepatitis B and C by Using Both Gradient-Recalled Echo and Spin-Echo Echo Planar Imaging: A Prospective Study. Am J Gastroenterol 2016;111:823-33. [Crossref] [PubMed]
- Chin JL, Pavlides M, Moolla A, et al. Non-invasive Markers of Liver Fibrosis: Adjuncts or Alternatives to Liver Biopsy? Front Pharmacol 2016;7:159. [Crossref] [PubMed]
- Liang XE, Zhong C, Huang L, et al. Stepwise Application of Transient Elastography and Routine Biomarkers Optimizes Hepatitis B Cirrhosis Detection. J Gastroenterol Hepatol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Lee JM, Seo YS, Kim TH, et al. The LAW index as an accurate indicator of the initiation of antiviral treatment in patients with chronic hepatitis B. J Gastroenterol Hepatol 2017;32:208-14. [Crossref] [PubMed]


