Gut microbiota and oxalate homeostasis
Introduction
Approximately 80% of stone forming individuals form calcium oxalate stones and hyperoxaluria is a major risk factor in this kidney disease (1,2). Oxalate is considered a useless end-product of mammalian metabolism and urinary oxalate is derived from endogenous metabolic sources, mainly produced by the liver (3), but also by dietary absorption of oxalate that can contribute as much as 50% of what is in urine (4). While urinary oxalate concentrations are in the millimolar range with an average of 0.25 mmoles/24 h in healthy controls, hyperoxaluria is defined by a urinary excretion greater than 0.45 mmoles/24 h (1,2,5). Blood levels of oxalate are several orders of magnitude lower and typically reported to be regulated at much less than 5 µM in normal healthy individuals (2,6-12). Thus, the epithelial barriers of the intestine and nephron mediate oxalate balance. In recent years, it has also been acknowledged that gut commensal bacteria with oxalate-degrading activity have the potential to contribute to oxalate homeostasis and this aspect is the focus of the current perspective. A number of published reviews with sub-sections on the role of oxalate-degrading bacteria are recommended reading, as background information, for the current perspective (13-18) which includes new and unpublished information from the author’s laboratory. This is not intended to be a review of the literature.
Physiological relevance of the oxalate-degrading commensal bacteria in rodents
Oxalobacter sp.
The most commonly described intestinal bacteria known to degrade oxalate are categorized into two groups: (I) the “generalist oxalotrophs”, including some strains of Bifidobacterium and Lactobacillus, that degrade alternative carbon sources in addition to oxalate; and (II) the “specialist oxalotrophs”, such as Oxalobacter formigenes, which is a commensal anaerobe that uses only oxalate as its sole carbon source (19,20). Investigations in a number of laboratories including ours, have confirmed that colonizing rats with Oxalobacter is consistently effective in reducing urinary oxalate excretion (21,22). A potential role for these bacteria in contributing to oxalate homeostasis is a reduction in the intraluminal oxalate load available for absorption across the intestine because of this oxalate-degrading action. In the mid-nineties, we provocatively proposed that, because of its substrate specificity, Oxalobacter may have a dual action of deriving oxalate from systemic sources, possibly deriving blood sources of its substrate by promoting enteric excretion of oxalate, in addition to intraluminal degradation. It is noteworthy that a compensatory pathway for enteric oxalate excretion was already documented by us and reported to occur in rats when renal function is compromised and this was attributed to activation of an angiotensin II (Ang II) signaling pathway in the large intestine (23,24). Subsequently, Oxalobacter-induced enteric oxalate excretion was confirmed for the first time in our laboratory using rats (21) and, in later studies, using mice (25,26). Notably, we have since determined that this Oxalobacter-induced enteric oxalate excretion is not mediated via Ang II since net oxalate secretion across colonized tissues was unaffected by the acute in vitro application of the specific Ang II receptor (AT1) antagonist, losartan, to the serosal bathing solution. The earlier study in rats (21) provided compelling evidence that the bacteria may produce a soluble secretagogue that initiates the enteric excretion of oxalate since oral administration of encapsulated bacterial lysate (cell membrane-free preparation) to rats produced changes in intestinal oxalate movements comparable to Oxalobacter colonization leading to reductions in urinary excretion. However, the precise signaling molecules and pathways involved in this physiological bacteria-host interaction have yet to be elucidated.
Remarkably, colonization of a mouse model [the alanine-glyoxylate aminotransferase (AGT) knockout] of the genetic disease Primary Hyperoxaluria, type 1 (PH1) with OXWR (a wild rat strain of Oxalobacter) resulted in a normalization of the hyperoxaluria and hyperoxalemia exhibited in non-colonized counterparts (26). This modulation of transmural oxalate transport was attributed to a physiological interaction between the bacteria and the enterocyte which promoted active transmural net oxalate secretion across the caecum and distal colon. Subsequent studies confirmed that the human Oxalobacter strain, HC-1, produced similar results in both the small and large intestine of wild type mice (25). Reductions in 24-h urinary oxalate excretion exceeding 90%, via the development of robust OXWR colonization, in mice are evident through time confirming the superior efficiency and efficacy of Oxalobacter formigenes in promoting enteric excretion and in degrading intraluminal oxalate (26).
Bifidobacterium sp.
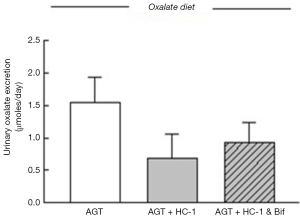
The modulation of intestinal oxalate transport by Oxalobacter prompted a study examining the effects of oxalate-degrading Bifidobacterium animalis compared to the non-degrader Bifidobacterium adolescentis since a 44% and 33% reduction in urinary excretion of oxalate in C57Bl/6 wild type and AGT knockout mice, respectively, was confirmed with B. animalis (27). The results showed that, in contrast to Oxalobacter sp., colonization of mice with Bifidobacterium sp. did not affect intestinal oxalate flux (27). Consequently, it was concluded that intraluminal oxalate degradation by B. animalis led to a reduction of the amount of oxalate available for absorption and lowered urinary oxalate. We subsequently sought to examine whether combined administration of HC-1 and B. animalis to mice would have a synergistic effect in reducing urinary oxalate excretion in AGT knockout mice. The protocol implemented in this study design was as previously described (25) except the gavage inoculum contained both HC-1 and B. animalis for one of the mouse groups and HC-1 alone for the other (n=6 in each group). Twelve days following the gavage procedure when mice were confirmed colonized, urine was collected for 24 h and the mice were then euthanized and oxalate fluxes were measured across the distal ileum, caecum, and distal colon. Compared to baseline values, urinary oxalate was found to be lower by 56% in the HC-1 treated animals compared to a 40% reduction in the group that received the combined inoculum (Figure 1). The flux results were surprising and revealed that the magnitude of net oxalate secretion promoted by HC-1 in all intestinal segments was attenuated somewhat when B. animalis was delivered in the combined inoculum (Figure 2). The magnitude of the net flux trended lower in all segments examined but reached significance only in the caecum due to a reduction in . As shown in Table 1, there were no changes in the associated electrical parameters of the tissue segments removed from both groups. This attenuated response in enteric oxalate excretion is reflected by the 16% higher urinary oxalate excretion in the mice that received the combined inoculum compared to HC-1 alone. A definitive explanation for this unexpected result is not readily available and warrants further study. It may be that competition for substrate, or indeed the establishment of a niche between both oxalate-degraders, are in play here. Given that B. animalis is a generalist oxalotroph, this versatile feature may endow some competitive advantage to this bacterial species over Oxalobacter in mouse intestine in vivo.

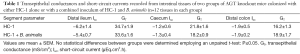
Full table
Lactobacillus sp.
More recently, we compared the effects of colonizing C57Bl/6 mice with two oxalate-degrading strains of Lactobacillus sp. on urinary oxalate excretion and intestinal oxalate transport in a similar study design as described for Bifidobacterium (27). The oxalate-degrading activity of the two Lactobacillus strains was previously documented (28) and confirmed in our hands prior to initiating the experimental design. The results of this study revealed that both L. acidophilus and L. gasseri comparably reduced 24-h urinary oxalate by 34% (from 1.1±0.3 to 0.7±0.1 µmoles/24 h, n=7) and 32% (from 1.4±0.2 to 0.9±0.3 µmoles/24 h, n=6), respectively. The flux studies examining oxalate movements across the distal colon (shown in Figure 3), caecum, and d-ileum (results not shown) removed from these mice exhibited no changes when compared to contemporary controls that were not administered either Lactobacillus strain. In addition, there were no changes in the associated electrical parameters of the tissue segments removed from the three animal groups (see Table 2). Thus, it was concluded that this effect of lowering urinary oxalate occurred as a consequence of a reduction in the availability of oxalate because of intraluminal oxalate degradation. Consequently, it appears that amongst the three oxalate-degrading bacterial species examined, Oxalobacter sp. is unique in modulating the intestinal transport of its sole carbon source which clearly has significant relevance from an evolutionary biology perspective.
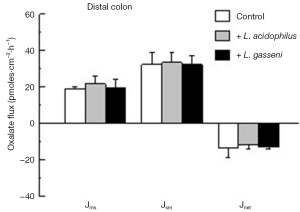

Full table
Probiotic oxalate-degrading bacteria in humans
In contrast to the studies in experimental animals, proof that these intestinal bacteria have a physiologically relevant role in contributing to oxalate homeostasis in humans has been more difficult to substantiate (14,17,29-38). This is discussed in a contemporary review (13) by this author emphasizing that “potential reasons for the conflicting and sometimes transient probiotic effects may include a lack of standardization of the various bacterial formulae and poor dietary control of oxalate and calcium intake in the experimental study design”. However, given the backdrop of reports showing human patients forming oxalate kidney stones, who are Oxalobacter-negative, have significantly higher plasma (37) and urinary oxalate excretion (37,39-42) and recurrent kidney stone episodes appear to correlate with the lack of Oxalobacter colonization (22,37,43,44), more rigorous design and implementation of human patient studies are required in order to reconcile the apparent beneficial probiotic affects in experimental animals compared to the conflicting and inconsistent results from studies in humans.
Oxalobacter colonization
Two studies that determined the prevalence of Oxalobacter colonization in the American population reported an overall prevalence of 38% in 247 adults between 18 and 69 years old (45) and 31% in 242 younger adults aged between 18 and 40 years (46) and it was noted that antibiotic consumption is a major factor impacting colonization status (45). In fact, very little is known about any of the factors that foster or repress the persistence of Oxalobacter colonization in humans or in experimental animals other than the sensitivity to certain antibiotics (47-49). Thus, there is a large gap in information and a lack of knowledge regarding this important aspect particularly in light of potential probiotic formulae under development. In laboratory animals, it has been generally acknowledged that, compared to their wild animal counterparts and due to breeding in specific pathogen-free (SPF) environments, these experimental models are not naturally colonized. However, our routine laboratory protocol tests every animal for the presence of Oxalobacter upon arrival into our animal holding facility and on two occasions, within the past 3 years, several C57BL/6 mice purchased from a well-known vendor were found to be colonized with Oxalobacter [detected by fresh fecal sample collection and inoculation into anaerobic media selective for oxalate-degrading activity (26) within 2 hours of receiving the mice into our mouse facility]. These fecal specimens were submitted for genomic sequencing at the University of Florida Core Services and results from the testing (qPCR of formyl-CoA transferase gene and sequencing 16S rRNA) confirmed that the oxalate-degrading bacteria were Oxalobacter. Another similar case was reported in 1987 by Daniel et al. who purchased rats from another vendor that were found to harbor oxalate-degrading bacteria (50). This underscores the importance of determining the Oxalobacter status of commercially available rodents prior to implementing experimental protocols on gut microbiota and oxalate homeostasis.
Another debatable aspect that we sought to address is the requirement to “prime” experimental rodents with an oxalate-supplemented diet prior to artificially colonizing the animals by an esophageal gavage with an Oxalobacter inoculum (26,51). Most investigators, including ourselves (21,25,26,52,53), have fed a “priming” diet since laboratory rodent chow appears to contain a nominal oxalate content. Support for feeding an oxalate priming diet prior to artificially colonizing rats was provided by Allison et al. who reported that the “establishment of a population of Oxalobacter in adult rats (that were not previously colonized) did not occur unless a relatively high level of sodium oxalate (3% or more) was added to the diet” (50,51). Further, Sidhu et al. reported that artificially colonized rats lose colonization within 5 days if dietary oxalate supplementation is stopped (54). We re-examined this supposition in mice by conducting a simple study involving 12 C57BL/6 mice. The mice, which were determined not to harbor Oxalobacter prior to study, were randomly divided into two groups, n=6 in each. One group was “primed’ by administering an oxalate-supplemented diet [1.5% (26)] for 5 days while the other was fed regular chow (Harlan Teklad #7912). Both groups were orally gavaged using the same HC-1 inoculum and protocol as previously described (26) and fresh fecal material was collected directly from all mice once a week for 77 days and inoculated into anaerobic media containing 20 mm oxalate for detection of oxalate-degrading activity (26). The oxalate-supplemented diet was replaced by regular chow in the “primed” group 12 days after the colonization procedure and both groups were fed the same rodent chow for the remainder of the study period. The results revealed unequivocally that at the end of the study period 100% of mice in both groups were found to be Oxalobacter-positive indicating that the provision of an oxalate-supplemented diet was not necessary for successful colonization of mice with Oxalobacter.
Clearly, the bioavailability of luminal oxalate will be a major factor in sustaining Oxalobacter colonization since this is the sole carbon source of these bacteria and we have shown that Oxalobacter can derive oxalate from systemic sources (21,25,26). However, other factors such as intraluminal calcium play an important role in so far as this ion impacts oxalate bioavailability by complexing with oxalate and forming the highly insoluble salt. In studies using rats, we have provided results showing that “the maintenance of Oxalobacter colonization appears to be exquisitely sensitive to the balance between intraluminal calcium and oxalate availability” (21). Also, in small study conducted in human individuals on controlled diets, Jiang et al. showed the impact of elevating intraluminal calcium 5-fold which resulted in reducing the number of fecal Oxalobacter by 5-fold (30). Conversely, the same study reported that increasing dietary oxalate 15-fold facilitated a 12-fold increase in fecal Oxalobacter (30). An interesting study on wild white-throated woodrats (Neotoma albigula) also reported that the impact of increasing dietary oxalate promotes complex interactions in the gut microbiome to include a beneficial effect on a community of bacteria involved directly/indirectly in luminal oxalate degradation. These physiologically relevant interactions within the gut microbiota certainly warrant further study in addition to studies on host-microbiota interactions in order to elucidate, as yet unknown, additional factors that impact Oxalobacter colonization.
Intestinal colonization of Oxalobacter is clearly affected by other unknown factors that are independent of luminal bioavailability of oxalate or intraluminal calcium (26) as we have found in studying the AGT knockout mouse model. This was evident in a study that compared artificially and naturally colonized AGT knockout mice administered diets with varying calcium content with/without oxalate (26). In every group of AGT knockout mice, colonization was lost within 3–4 weeks compared to the retention of colonization in the control group (26). The results of several small studies of PH1 patients administered Oxalobacter generally showed transient reductions in urinary oxalate excretion that were reversed when probiotic treatment was terminated suggesting colonization was not achieved by the treatment (29,33,34). Whether these patients have an inherently hostile intraluminal environment with respect to Oxalobacter colonization, similar to what is observed in the animal model of this genetic disease, is purely speculative but warrants study since this patient group, in particular, would benefit greatly from a probiotic therapeutic approach.
Another learning opportunity regarding intestinal Oxalobacter colonization is presented by examining the heterogeneity of the different intestinal segments that support the presence of Oxalobacter for varying periods of time (25). A study conducted by us in wild type mice showed that Oxalobacter can transiently reside in the small intestine, specifically in the proximal jejunum, mid- and distal-ileum in some mice. In contrast, sustained colonization was observed in all mice in the large intestine. Given the large variations in pH as well as in the general chemical milieu and composition of the microbiome of each of these segments, addressing these questions presents a complex and challenging task. Add to this, the heterogeneity of the segment specific oxalate transport proteins sub-serving the basal characteristics of oxalate movements in each segment and numerous other questions arise. Among the most basic of questions, however, is the physical location of Oxalobacter with respect to the apical surface of the enterocyte? Where in that microenvironment does Oxalobacter reside and what are the microenvironmental factors that impact retention of this oxalotrophic specialist? If the mechanism whereby Oxalobacter modifies epithelial oxalate transport is via the elaboration of a secretagogue as previously proposed (21), the nature of the microclimate and distance influencing the diffusion of this purported secretagogue is relevant information.
A final consideration affecting Oxalobacter colonization is the destabilization of the intestinal microbiome with the introduction of different feeds and new environments. This is not a trivial issue as we have experienced upon moving our experimental mice from an older facility to a newly built facility at our institution some years ago. This seldom acknowledged factor was elegantly addressed experimentally by Ussar et al. who demonstrated that “the composition of microbiota is highly dependent on diet, environmental history, and host genetics” (55) due to remodeling of gut microbiota. This presents a quagmire when trying to reconcile inconsistencies in results within and between different laboratories and underscores the importance of each investigative laboratory to normalize their experimental animals with respect to these factors.
Acknowledgements
The author thanks Heran Getachew and Morgan Parker for excellent technical assistance in contributing to the studies presented herein. The generosity of Dr. Allison at Iowa State University is also acknowledged for providing Oxalobacter formigenes sp.
Funding: This work was supported by the National Institutes of Health (DK088892 to M. H.).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: All applicable international, national, and institutional guidelines for the care and use of animals in the studies included in this report were followed. This article does not contain any studies with human participants performed by the author.
References
- Worcester EM, Coe FL. Nephrolithiasis. Prim Care 2008;35:369-91. vii. [Crossref] [PubMed]
- Hatch M. Oxalate status in stone-formers. Two distinct hyperoxaluric entities. Urol Res 1993;21:55-9. [Crossref] [PubMed]
- Holmes RP, Assimos DG. Glyoxylate synthesis, and its modulation and influence on oxalate synthesis. J Urol 1998;160:1617-24. [Crossref] [PubMed]
- Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int 2001;59:270-6. [Crossref] [PubMed]
- Kasidas GP. Assay of oxalate and glycollate in urine. In: Rose GA. editor. Oxalate metabolism in relation to urinary stone. Berlin Heidelberg New York: Springer-Verlag, 1988:7-26.
- Harris AH, Freel RW, Hatch M. Serum oxalate in human beings and rats as determined with the use of ion chromatography. J Lab Clin Med 2004;144:45-52. [Crossref] [PubMed]
- Wilson DM, Liedtke RR. Modified enzyme-based colorimetric assay of urinary and plasma oxalate with improved sensitivity and no ascorbate interference: reference values and sample handling procedures. Clin Chem 1991;37:1229-35. [PubMed]
- Petrarulo M, Cerelli E, Marangella M, et al. Assay of plasma oxalate with soluble oxalate oxidase. Clin Chem 1994;40:2030-4. [PubMed]
- Hönow R, Simon A, Hesse A. Interference-free sample preparation for the determination of plasma oxalate analyzed by HPLC-ER: preliminary results from calcium oxalate stone-formers and non-stone-formers. Clin Chim Acta 2002;318:19-24. [Crossref] [PubMed]
- Hatch M. Spectrophotometric determination of oxalate in whole blood. Clin Chim Acta 1990;193:199-202. [Crossref] [PubMed]
- Rolton HA, McConnell KN, Modi KS, et al. A simple, rapid assay for plasma oxalate in uraemic patients using oxalate oxidase, which is free from vitamin C interference. Clin Chim Acta 1989;182:247-54. [Crossref] [PubMed]
- Kasidas GP, Rose GA. Measurement of plasma oxalate in healthy subjects and in patients with chronic renal failure using immobilised oxalate oxidase. Clin Chim Acta 1986;154:49-58. [Crossref] [PubMed]
- Whittamore JM, Hatch M. The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Hatch M, Freel RW. The roles and mechanisms of intestinal oxalate transport in oxalate homeostasis. Semin Nephrol 2008;28:143-51. [Crossref] [PubMed]
- Knight J, Deora R, Assimos DG, et al. The genetic composition of Oxalobacter formigenes and its relationship to colonization and calcium oxalate stone disease. Urolithiasis 2013;41:187-96. [Crossref] [PubMed]
- Asplin JR. The management of patients with enteric hyperoxaluria. Urolithiasis 2016;44:33-43. [Crossref] [PubMed]
- Liebman M, Al-Wahsh IA. Probiotics and other key determinants of dietary oxalate absorption. Adv Nutr 2011;2:254-60. [Crossref] [PubMed]
- Abratt VR, Reid SJ. Oxalate-degrading bacteria of the human gut as probiotics in the management of kidney stone disease. Adv Appl Microbiol 2010;72:63-87. [Crossref] [PubMed]
- Allison MJ, Dawson KA, Mayberry WR, et al. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol 1985;141:1-7. [Crossref] [PubMed]
- Dawson KA, Allison MJ, Hartman PA. Characteristics of anaerobic oxalate-degrading enrichment cultures from the rumen. Appl Environ Microbiol 1980;40:840-6. [PubMed]
- Hatch M, Cornelius J, Allison M, et al. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int 2006;69:691-8. [Crossref] [PubMed]
- Sidhu H, Schmidt ME, Cornelius JG, et al. Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J Am Soc Nephrol 1999;10 Suppl 14:S334-40. [PubMed]
- Hatch M, Freel RW. Angiotensin II involvement in adaptive enteric oxalate excretion in rats with chronic renal failure induced by hyperoxaluria. Urol Res 2003;31:426-32. [Crossref] [PubMed]
- Hatch M, Freel RW, Vaziri ND. Regulatory aspects of oxalate secretion in enteric oxalate elimination. J Am Soc Nephrol 1999;10 Suppl 14:S324-8. [PubMed]
- Hatch M, Freel RW. A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis 2013;41:379-84. [Crossref] [PubMed]
- Hatch M, Gjymishka A, Salido EC, et al. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol 2011;300:G461-9. [Crossref] [PubMed]
- Klimesova K, Whittamore JM, Hatch M. Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis 2015;43:107-17. [Crossref] [PubMed]
- Turroni S, Vitali B, Bendazzoli C, et al. Oxalate consumption by lactobacilli: evaluation of oxalyl-CoA decarboxylase and formyl-CoA transferase activity in Lactobacillus acidophilus. J Appl Microbiol 2007;103:1600-9. [Crossref] [PubMed]
- Hoppe B, Groothoff JW, Hulton SA, et al. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transplant 2011;26:3609-15. [Crossref] [PubMed]
- Jiang J, Knight J, Easter LH, et al. Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol 2011;186:135-9. [Crossref] [PubMed]
- Lieske JC, Tremaine WJ, De Simone C, et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int 2010;78:1178-85. [Crossref] [PubMed]
- Siener R, Bade DJ, Hesse A, et al. Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. J Transl Med 2013;11:306. [Crossref] [PubMed]
- Hoppe B, von Unruh G, Laube N, et al. Oxalate degrading bacteria: new treatment option for patients with primary and secondary hyperoxaluria? Urol Res 2005;33:372-5. [Crossref] [PubMed]
- Hoppe B, Beck B, Gatter N, et al. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int 2006;70:1305-11. [Crossref] [PubMed]
- Lieske JC, Goldfarb DS, De Simone C, et al. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int 2005;68:1244-9. [Crossref] [PubMed]
- Goldfarb DS, Modersitzki F, Asplin JR. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin J Am Soc Nephrol 2007;2:745-9. [Crossref] [PubMed]
- Siener R, Bangen U, Sidhu H, et al. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int 2013;83:1144-9. [Crossref] [PubMed]
- Campieri C, Campieri M, Bertuzzi V, et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int 2001;60:1097-105. [Crossref] [PubMed]
- Neuhaus TJ, Belzer T, Blau N, et al. Urinary oxalate excretion in urolithiasis and nephrocalcinosis. Arch Dis Child 2000;82:322-6. [Crossref] [PubMed]
- Troxel SA, Sidhu H, Kaul P, et al. Intestinal Oxalobacter formigenes colonization in calcium oxalate stone formers and its relation to urinary oxalate. J Endourol 2003;17:173-6. [Crossref] [PubMed]
- Kwak C, Kim HK, Kim EC, et al. Urinary oxalate levels and the enteric bacterium Oxalobacter formigenes in patients with calcium oxalate urolithiasis. Eur Urol 2003;44:475-81. [Crossref] [PubMed]
- Mikami K, Akakura K, Takei K, et al. Association of absence of intestinal oxalate degrading bacteria with urinary calcium oxalate stone formation. Int J Urol 2003;10:293-6. [Crossref] [PubMed]
- Kumar R, Mukherjee M, Bhandari M, et al. Role of Oxalobacter formigenes in calcium oxalate stone disease: a study from North India. Eur Urol 2002;41:318-22. [Crossref] [PubMed]
- Mittal RD, Kumar R, Mittal B, et al. Stone composition, metabolic profile and the presence of the gut-inhabiting bacterium Oxalobacter formigenes as risk factors for renal stone formation. Med Princ Pract 2003;12:208-13. [Crossref] [PubMed]
- Kelly JP, Curhan GC, Cave DR, et al. Factors related to colonization with Oxalobacter formigenes in U.S. adults. J Endourol 2011;25:673-9. [Crossref] [PubMed]
- Barnett C, Nazzal L, Goldfarb DS, et al. The Presence of Oxalobacter formigenes in the Microbiome of Healthy Young Adults. J Urol 2016;195:499-506. [Crossref] [PubMed]
- Lange JN, Wood KD, Wong H, et al. Sensitivity of human strains of Oxalobacter formigenes to commonly prescribed antibiotics. Urology 2012;79:1286-9. [Crossref] [PubMed]
- Mittal RD, Kumar R, Bid HK, et al. Effect of antibiotics on Oxalobacter formigenes colonization of human gastrointestinal tract. J Endourol 2005;19:102-6. [Crossref] [PubMed]
- Kharlamb V, Schelker J, Francois F, et al. Oral antibiotic treatment of Helicobacter pylori leads to persistently reduced intestinal colonization rates with Oxalobacter formigenes. J Endourol 2011;25:1781-5. [Crossref] [PubMed]
- Daniel SL, Hartman PA, Allison MJ. Microbial degradation of oxalate in the gastrointestinal tracts of rats. Appl Environ Microbiol 1987;53:1793-7. [PubMed]
- Allison MJ, Daniel SL, Cornick NA. Oxalate-degrading Bacteria. In: Khan SR. editor. Calcium Oxalate in Biological Systems. Boca Raton New York London Tokyo: CRC Press, 1995:131-68.
- Canales BK, Hatch M. Kidney stone incidence and metabolic urinary changes after modern bariatric surgery: review of clinical studies, experimental models, and prevention strategies. Surg Obes Relat Dis 2014;10:734-42. [Crossref] [PubMed]
- Hatch M, Canales BK. The mechanistic basis of hyperoxaluria following gastric bypass in obese rats. Urolithiasis 2016;44:221-30. [Crossref] [PubMed]
- Sidhu H, Allison MJ, Chow JM, et al. Rapid reversal of hyperoxaluria in a rat model after probiotic administration of Oxalobacter formigenes. J Urol 2001;166:1487-91. [Crossref] [PubMed]
- Ussar S, Griffin NW, Bezy O, et al. Interactions between Gut Microbiota, Host Genetics and Diet Modulate the Predisposition to Obesity and Metabolic Syndrome. Cell Metab 2015;22:516-30. [Crossref] [PubMed]


