Targeting NF2-Hippo/Yap signaling pathway for cardioprotection after ischemia/reperfusion injury
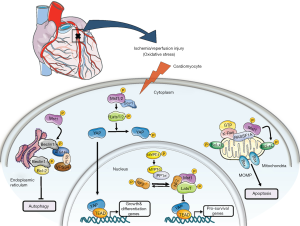
Cardiac ischemia/reperfusion (I/R) injury is one of the most deleterious cardiovascular condition and a leading cause of mortality worldwide. The deprivation of oxygen and nutrient supply, due to blockage of coronary arteries, causes an oxidative stress on the myocardium leading to an irreversible loss of cardiomyocytes and impaired cardiac output. Timely reperfusion of coronary flow is necessary to reduce the oxidative stress and to limit myocardial injury. However, the process of reperfusion can itself induce cardiomyocyte death, known as myocardial reperfusion injury. Recently, a series of studies have implicated Hippo signaling pathway in cardiomyocyte proliferation and survival during and after myocardial I/R injury (Figure 1) (1-4). However, the upstream modulators of Hippo-Yap pathway during myocardial I/R injury have not been identified.
The Hippo signaling pathway, an evolutionarily conserved pathway, was first described in Drosophila melanogaster as a major regulator of tissue growth and organ size. The Hippo signaling pathway controls organ size by regulating cell proliferation, cell survival, and stem cell self-renewal (5). The core components of Hippo pathway consists of a serine/threonine kinase cascade, transcriptional coactivators and transcription factors. Activation of upstream kinases such as mammalian sterile 20-like protein kinase (Mst1/2), large tumor suppressor (Lats1/2) and adaptor protein Salvador (Salv) promotes phosphorylation of major downstream transcriptional regulators Yap and Taz, resulting in its cytoplasmic retention. In contrast, when upstream kinases are inactivated, unphosphorylated Yap and Taz can translocate into the nucleus and binds to various transcription factors including Tead1-4 to regulate transcriptional activity of genes involved in cell proliferation and survival (Figure 1) (2,5).
Genetic studies in mice have implicated Hippo signaling pathway in cardiovascular development as well as in cardiac repair and regeneration after myocardial I/R injury (1,3,6-8). Cardiomyocyte specific genetic deletion of upstream kinases Mst1/2, Lats2 or Salv leads to cardiac hyperplasia due to increase in cell proliferation (7). In contrast, deletion of Yap leads to cardiac hypoplasia due to impaired cell proliferation (4,8). Interestingly, the Hippo pathway restrains cardiomyocyte proliferation during embryonic development by modulating Yap interaction with insulin-like growth factor (IGF) and Wnt signaling pathways (7,8). Deregulation of hippo pathway in adult heart has been associated with numerous cardiac abnormalities including cardiac failures and ischemic cardiac disease. Yap and Taz are required in a dose dependent manner for cardiac regeneration (3). Cardiomyocyte-specific overexpression of a constitutively active Yap promotes cardiac regeneration after myocardial injury in part by stimulating IGF and Akt signaling (3). In consistent with this, activation of endogenous Yap (in hippo kinase Salv, Lats1 and Lats2-deficient hearts) also promotes cardiac regeneration and protect against myocardial injury (1). Mst1 is activated in cardiac myocytes in response to oxidative stress after I/R injury (9). Mst1 activation after I/R injury is not only associated with increased myocyte death but also decreased autophagy (Figure 1) (9-11). Cardiomyocyte-specific Mst1 overexpression resulted in cardiac dysfunction (9). However inhibition of endogenous Mst1, using a dominant negative Mst1, prevented cardiomyocytes death and improved cardiac function after myocardial injury (9,12). The canonical/non-canonical regulation of upstream and downstream components of hippo pathway might lead to alternative cellular outcomes during heart regeneration/injury, especially with respect to the cardiomyocytes. For instance, stress induced activation of Mst1 leads to phosphorylation of Lats2, which form the canonical hippo pathway together with Yap, and regulate cardiomyocyte proliferation during embryonic development and regeneration (Figure 1) (1,3,8). Importantly, activated Mst1 also phosphorylate substrates outside of canonical hippo pathway, such as Bcl-xL proteins in the mitochondria and promote cardiomyocytes death (9,12). Mst1 also phosphorylates Beclin1 in the endoplasmic reticulum and suppresses autophagy during I/R injury (Figure 1) (11). Thus, identifying upstream regulators of hippo signaling that could precisely activate different components of hippo pathway in the cardiomyocytes, for alternative cellular outcomes, will help to design strategies to target it pharmacologically and to improve cardiac function after I/R injury.
Several signals have been identified that can activate/regulate the hippo-pathway such as oxidative stress, mechanical stress and pathological stress such as cancer. In mammals, most members of the hippo signaling pathway including Mst1/2 and Lats1/2 kinases, and the Salv protein are considered to act as tumor suppressors because mutations in these components result in an overgrowth phenotype. Neurofibromin 2 (NF2), also called merlin, has been implicated as one of the upstream regulator of the hippo signaling pathway in cancers. Recent studies have demonstrated that liver-specific inactivation of NF2 induces hepatocellular carcinoma and such effect can be suppressed by the deletion of Yap, suggesting an important link between NF2 and the hippo-pathway effector molecule (13,14). In another study, authors demonstrated that NF2 mediated inhibition of Yap and Taz is required for neural progenitor expansion during brain development (15). NF2 is a member of neurofibromin family (NF1 and NF2), a GTPase-activating protein that regulates RAS signaling pathway and thereby plays a role in intra and extra-cellular signal transduction necessary for cell proliferation and differentiation in tumor pathology. Besides cancer, the loss of NF1 has been shown to trigger the RAS signaling pathway in several cardiovascular diseases including cardiac hypertrophy, cardiomyopathy, and cardiac fibrosis (16-18). In contrast to NF1, role of NF2 in the cardiovascular system is poorly understood.
In a recent study, Matsuda et al. identified NF2 as a regulator of hippo signaling pathway in cardiomyocyte after I/R injury (19). Authors demonstrated that NF2 is activated in cardiomyocytes/myocardium in response to oxidative stress and exacerbate I/R injury by promoting Mst1-mediated cardiomyocyte apoptosis. Myosin phosphatase target subunit 1 (MYPT-1), a component of protein phosphatase is activated after oxidative stress caused by I/R injury, leading to dephosphorylation (activation) of NF2 in cardiomyocytes (Figure 1). Activated NF2 promote nuclear localization of Mst1 in cardiomyocytes. Further experiments suggested that activated NF2 physically interact with Mst1 and Lats2 in heart subjected to I/R injury and regulate cardiomyocyte apoptosis. Knockdown of either MYPT-1 or NF2 using siRNA decreased Mst1-mediated cardiomyocyte apoptosis in vitro (Figure 1).
Furthermore, the authors took a genetic approach to demonstrate the cardioprotective effects of NF2 after I/R injury. Authors genetically deleted NF2 using a cardiomyocyte-specific αMHC-Cre transgenic mice (CreαMHC; NF2flox/flox) and subjected to I/R injury ex vivo and in vivo. In both conditions, cardiomyocyte-specific NF2 knockouts showed protection, as the infarcted myocardial region was significantly smaller than controls and improved cardiac function after I/R injury. Mechanistically, the cardioprotective effect of NF2 deletion was explained by activation of Yap and its target genes such as ctgf, cyr61, fgf2 and birc5. Finally, using a genetic approach this study further linked the importance of Yap activation in NF2 deficient heart after I/R injury. For example, genetic inhibition of Yap in a NF2 knockout background (Creα-MHC; Yap flox/+; NF2flox/flox) abolished cardioprotection observed in NF2 knockout hearts (Creα-MHC; NF2flox/flox), evident by increased apoptosis and infract size. Together, this study demonstrates the critical role of NF2 in aggravating I/R injury by activating Mst1 and inhibiting Yap in cardiomyocyte (Figure 1).
Many studies have implicated Hippo signaling in cardiac development, neonatal and adult cardiomyocyte cell proliferation and regenerative response after cardiac injury (1-8). Inhibiting upstream kinases or activation of Yap have shown to have beneficial outcomes in cardiac injury. Several reports on ischemic myocardial injury have demonstrated a YAP-mediated survival and proliferation of cardiomyocytes, a process that seems to be dependent on the AKT activation, which eventually confers protection against myocardial injury in response to stress. In contrast, a recent study on ischemic acute kidney injury has demonstrated that a continuous activation of YAP might facilitate pro-fibrotic outcomes that ultimately results in interstitial fibrosis (20). Therefore, understanding a tissue specific regulation of the hippo pathway might help to design potential therapies to treat ischemic tissue injury.
Acknowledgements
Funding: This work was supported by funds from Duke-NUS Medical School Singapore, Goh foundation and Singapore NRF fellowship (NRF-NRFF2016-01) to MK Singh.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heallen T, Morikawa Y, Leach J, et al. Hippo signaling impedes adult heart regeneration. Development 2013;140:4683-90. [Crossref] [PubMed]
- Zhou Q, Li L, Zhao B, et al. The hippo pathway in heart development, regeneration, and diseases. Circ Res 2015;116:1431-47. [Crossref] [PubMed]
- Xin M, Kim Y, Sutherland LB, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA 2013;110:13839-44. [Crossref] [PubMed]
- von Gise A, Lin Z, Schlegelmilch K, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci USA 2012;109:2394-9. [Crossref] [PubMed]
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 2011;13:877-83. [Crossref] [PubMed]
- Singh A, Ramesh S, Cibi DM, et al. Hippo Signaling Mediators Yap and Taz Are Required in the Epicardium for Coronary Vasculature Development. Cell Rep 2016;15:1384-93. [Crossref] [PubMed]
- Heallen T, Zhang M, Wang J, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011;332:458-61. [Crossref] [PubMed]
- Xin M, Kim Y, Sutherland LB, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal 2011;4:ra70. [Crossref] [PubMed]
- Yamamoto S, Yang G, Zablocki D, et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest 2003;111:1463-74. [Crossref] [PubMed]
- Del Re DP, Matsuda T, Zhai P, et al. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol Cell 2014;54:639-50. [Crossref] [PubMed]
- Maejima Y, Kyoi S, Zhai P, et al. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat Med 2013;19:1478-88. [Crossref] [PubMed]
- Odashima M, Usui S, Takagi H, et al. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ Res 2007;100:1344-52. [Crossref] [PubMed]
- Benhamouche S, Curto M, Saotome I, et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev 2010;24:1718-30. [Crossref] [PubMed]
- Zhang N, Bai H, David KK, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell 2010;19:27-38. [Crossref] [PubMed]
- Lavado A, He Y, Paré J, et al. Tumor suppressor Nf2 limits expansion of the neural progenitor pool by inhibiting Yap/Taz transcriptional coactivators. Development 2013;140:3323-34. [Crossref] [PubMed]
- Brannan CI, Perkins AS, Vogel KS, et al. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev 1994;8:1019-29. [Crossref] [PubMed]
- Gitler AD, Zhu Y, Ismat FA, et al. Nf1 has an essential role in endothelial cells. Nat Genet 2003;33:75-9. [Crossref] [PubMed]
- Xu J, Ismat FA, Wang T, et al. Cardiomyocyte-specific loss of neurofibromin promotes cardiac hypertrophy and dysfunction. Circ Res 2009;105:304-11. [Crossref] [PubMed]
- Matsuda T, Zhai P, Sciarretta S, et al. NF2 Activates Hippo Signaling and Promotes Ischemia/Reperfusion Injury in the Heart. Circ Res 2016;119:596-606. [Crossref] [PubMed]
- Xu J, Li PX, Wu J, et al. Involvement of the Hippo pathway in regeneration and fibrogenesis after ischaemic acute kidney injury: YAP is the key effector. Clin Sci (Lond) 2016;130:349-63. [Crossref] [PubMed]