Companion diagnostics—a tool to improve pharmacotherapy
Introduction
Over the years, several publications have drawn our attention to the variability of pharmacotherapy, which in many cases can be of a significant magnitude (1-3). The main contributor to this variability is diseases heterogeneity, and patients who have similar diagnoses very often respond differently to the same pharmacological intervention, with great variability in both efficacy and safety outcome. Despite having discussed personalized medicine for more than a decade, we still see that most drug prescriptions are largely based on ‘trial and error’ and not on solid biomarker data (1,4,5). For serious chronic diseases, such an approach can have unfortunate medical consequences for the individual patients. However, with the advance of molecular diagnostics and subsequently an increased understanding of disease mechanisms, things are slowly changing. Within the last few years, we have seen an increasing number of predictive biomarker assays being developed to guide the use of targeted cancer drugs. This type of assay is called companion diagnostics and is most often developed in parallel to the drug using the drug-diagnostic co-development model (6). For a number of these drugs the companion diagnostics have taken up a central role in the development process, and the success of this type of targeted therapy largely depends on the performance of the assays.
At the recent 4th Joint Congress of the International Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and the European Union of Medical Specialists (UEMS) in Warsaw, Poland, the first author of this article gave a plenary lecture entitled “Clinical Application of Companion Diagnostics” (7). The current article is primarily based on this presentation and summarizes some of the recent developments within the fast evolving area of companion diagnostics and drug-diagnostic co-development.
Companion diagnostics in a historical perspective
Looking at the history of companion diagnostics, the first time we see molecular testing integrated in the drug development process was in the 1970s. When the selective estrogen receptor modulator tamoxifen (Nolvadex, AstraZeneca) was developed for the treatment of advanced breast cancer, and here data on estrogen receptor (ER) status was correlated with treatment outcome. Based on data from a phase II study in patients with advanced stage breast cancer, published in 1976, the investigators concluded: ‘A high degree of correlation between response and positive estrogen-receptor assay suggests the value of the diagnostic test as a means to select patients for tamoxifen treatment’ (8,9). Despite the fact that this study was published 40 years ago these principles still apply when drug and diagnostic are developed in parallel. However, in the described phase II study, testing for ER status was not performed prospectively, and it was not until a decade later that the drug-diagnostic co-development model really proved its value.
In the 1980’s, the US scientist Dennis J. Slamon discovered the link between amplification of the HER2 gene and poor disease prognosis in breast cancer, which lead him to suggest the development of a specific HER2 antagonist (10). This antagonist became the monoclonal antibody trastuzumab (Herceptin, Roche/Genentech), and when Genentech developed this drug for treatment of metastatic breast cancer they developed a clinical trial assay simultaneously. This assay was an immunohistochemistry (IHC) assay for determination of tumor HER2 overexpression. When Genentech took trastuzumab into clinical development they used this assay to preselect the patients for treatment with their drug, and in fact, they formed the basis for the enrichment study design as we know it today. The diagnostic company Dako further improved this IHC assay, which today is known as the HercepTest, and in September 1998 the US Food and Drug Administration (FDA) simultaneously granted approval of drug and diagnostic (11,12). HercepTest became the first companion diagnostic assay linked to the use of a specific drug, and the way that Genentech did the parallel development of drug and diagnostic has served as an inspiration to a number of other pharmaceutical and biotech companies as well as regulatory agencies. In 2005 when the US FDA issued their concept paper on drug-diagnostic co-development, the inspiration was likewise clear (13).
Over the past 10–20 years an increasing number of drug-diagnostic combinations have obtained regulatory approval. Figure 1 gives an overview of this development, beginning with the development of tamoxifen for treatment of advanced breast cancer, in the 1970’s, to the recent approval of the PD-L1 checkpoint inhibitor atezolizumab (Tecentriq, Roche/Genentech). When atezolizumab was approved by the US FDA a simultaneous approval was obtained for its complementary diagnostic assay [Ventana PD-L1 (SP142) assay] (14-16).

Definitions of companion diagnostics
In the years following the approval of the HercepTest this type of predictive biomarker assay was referred to as pharmacodiagnostics, theranostics or pharmacogenetics. The term companion diagnostic appears for the first time in the literature in an article published in Nature Biotechnology in 2006 (17). Here, the authors stated that this type of assay could simplify the drug discovery process, make clinical trials more efficient and informative as well as be used to individualize therapy. Now, companion diagnostics is also the term that the different regulatory authorities have adapted to describe a predictive biomarker assay developed in parallel to a specific drug (18,19).
In 2014, the US FDA issued a guidance document on In Vitro Companion Diagnostics Devices in which they defined what a companion diagnostic is (18). According to this definition, a companion diagnostic assay is an in vitro diagnostic device that provides information that is essential for the safe and effective use of a corresponding therapeutic product. Furthermore, the US FDA specifies four areas, where a companion diagnostic assay could be essential: (I) to identify patients who are most likely to benefit from the therapeutic product; (II) to identify patients likely to be at increased risk of serious adverse reactions as a result of treatment with the therapeutic product; (III) to monitor response to treatment with the therapeutic product for the purpose of adjusting treatment (e.g., schedule, dose, discontinuation) to achieve improved safety or effectiveness; and finally; (IV) to identify patients in the population for whom the therapeutic product has been adequately studied, and found safe and effective, i.e., there is insufficient information about the safety and effectiveness of the therapeutic product in any other population. Overall, the US FDA definition can be summarized as outcome prediction (efficacy and safety) as well as therapy monitoring. The fourth item mentioned in the definition can be regarded as a kind of disclaimer, which is related to the type of study design most often used in the clinical validation of companion diagnostic assays; the enrichment or targeted study design.
Until now no official definition of what a companion diagnostic is has been made in the European Union (EU). However, this will soon change with the implementation of the new legislation on in vitro diagnostic medical devices (19). According to the draft published in June 2016 the definition of a companion diagnostic assay is somewhat different compared to the 2014 guidance document on In Vitro Companion Diagnostics Devices issued by the US FDA. In the new EU regulation, it is stated that companion diagnostics are essential to define a patient’s eligibility to a specific treatment with a medicinal product through the quantitative or qualitative determination of specific markers identifying subjects at higher risk of developing adverse reaction to the specific medicinal product, or identifying patients in the population for whom the therapeutic product has been adequately studied, and found safe and effective. Such biomarker(s) may be present in healthy subjects and/or in patients. Furthermore, it is stated that devices used to monitoring a treatment with a medicinal product in order to ensure the concentration of relevant substances in the human body is within the therapeutic window are not considered companion diagnostics. Especially the last part of the EU definition is different compared to the US one, where monitoring of response to treatment with a medical product is included in the definition of a companion diagnostic assay.
Drug-diagnostic co-development
Companion diagnostic assays are most often developed in parallel to a drug, using the drug diagnostic co-development model. The success of this model depends on the strength of the biomarker hypothesis deduced during the early research and preclinical phases of the drug development. The generation of a solid hypothesis requires a thorough molecular understanding of both the disease biology and the drug mechanism of action (4,7). Based on this hypothesis a prototype assay is developed, which is then tested in the early phase of the clinical development in order to assess the predictive potential. If such a potential exists the next step is the analytical validation; however, before this part can be finalized the clinical cut-off must be selected. The selection of cut-off can be a very challenging exercise, due to the often limited clinical outcome data available at this early stage of development.
How critical it is to select the right cut-off has been demonstrated very recently with respect to the PD-1 immune checkpoint inhibitors pembrolizumab (Keytruda, Merck Sharp & Dohme) and nivolumab (Opdivo, Bristol-Myers Squibb) in relation to first-line treatment of advanced stage non-small cell lung cancer (NSCLC) (20-22). The companion diagnostic (PD-L1 IHC 22C3 pharmDx, Dako) linked to pembrolizumab has a cut-off of 50% PD-L1 expression whereas this is only 5% for the assay (PD-L1 IHC 28-8 pharmDx, Dako) linked to nivolumab. At the recent ESMO Congress in Copenhagen data with both immune checkpoint inhibitors was presented, and here, nivolumab, in the CheckMate 026 study, failed to demonstrate superiority over platinum based chemotherapy (22). Despite it is not possible to make a direct comparison between the two drug-diagnostic combination, a likely explanation for the failure of nivolumab is the lower assay cut-off value that allow a wider patient population to be treated with this drug. As this recent example shows, the selection of the right clinical cut-off for a companion diagnostic assay can in fact be a question about success or failure for the drug it is meant to guide. Despite the different IHC PD-L1 assays seeming to lack sufficient sensitivity, they have to some extent been able to predict the response to treatment with the different immune checkpoint inhibitors. This is particularly true for patients with high expression levels of PD-L1 in NSCLC, urothelial carcinoma and melanoma (23-25).
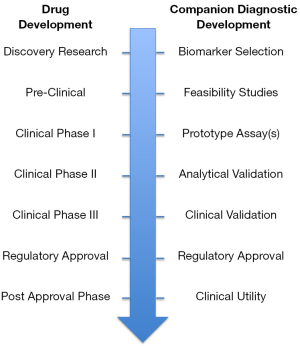
When the clinical cut-off has been selected the analytical validation of the companion diagnostic assay can be finalized. The validation must demonstrate that the assay is robust and reliable. It does not matter that a strong biomarker hypothesis has been generated and the prototype assay has demonstrated a predictive potential during early clinical development, if the results cannot be trusted. When the analytical validation has been concluded successfully the final clinical validation can be initiated. Here, the companion diagnostic assay must demonstrate its ability to stratify the patients into likely responders or likely non-responders, and subsequently also show that the group of patients that was characterized as likely responders were also the ones that benefitted the most from the treatment with the drug. The clinical validation of the assay is normally performed in the late clinical phases, phase IIb or phase III, at the same time as safety and efficacy are demonstrated for the drug. If the goals are fulfilled with respect to safety and efficacy for the drug-diagnostic combination, a simultaneous regulatory approval will most likely be granted for both. This simultaneous approval process makes sense, due to the fact that the analytical and clinical validated companion diagnostic assay need to be available at the same time as the drug, in order to guide its use (7). Figure 2 summarizes the different major steps in the development of a companion diagnostic assay, from biomarker discovery to regulatory approval.
Over the past 5 to 10 years we have witnessed a number of drug development programs within oncology that have been concluded successfully and where companion diagnostic assays have played a crucial role. One such example is the development of the ALK inhibitor crizotinib (Xalkori, Pfizer) for treatment of NSCLC. Here, the concurrently developed ALK break-apart FISH assay (Vysis ALK Break Apart FISH Probe Kit, Abbott Molecular) was used to detect ALK-rearrangement and thereby enriching the study population with biomarker positive patients only. Two open, non-randomized studies with a total of 255 ALK-positive patients were sufficient to demonstrate efficacy of crizotinib and to obtain US FDA approval (26). After the approval in 2011 of crizotinib, two other ALK inhibitors have subsequently been approved for treatment of advanced stage ALK-positive NSCLC, and here, the clinical development programs were based on even fewer patients. However, the recent approval of crizotinib for treatment of NSCLC patients with ROS1-rearrangement was even lower as regards to the number of patients. Here, only 50 ROS1-positive metastatic NSCLC patients treated in one single-arm study were sufficient to demonstrate efficacy and obtain regulatory approval by the US FDA (27). The objective response rate in this group of patients was close to 70% with a median duration of response of more than 18 months, which is quite impressive in patients with metastatic NSCLC (28,29). Such results are only obtainable if there is a thorough molecular understanding of the pathophysiology and the mechanism of action of the drug, and this knowledge is “translated” into a practical usable companion diagnostic that is able to select the group of likely responding patients. Table 1 gives an overview of the ALK and ROS1 inhibitors approved so far and the number of patients and clinical studies that was needed to demonstrate efficacy of the individual drugs.

Full table
An objective response rate in the range of 60%–70%, as described above for crizotinib in patients with ROS1-rearrangement, is an impressive outcome in patients with metastatic NSCLC. However, looking at the results from other oncology drug development programs, where the drug-diagnostic co-development model has been used, this is not outstanding. In Table 2 are listed some of these drugs that all have obtained approval within the past 15 years for different advanced or metastatic cancer indications. The objective response rates listed in the table are extracted from the prescribing information available to healthcare professionals in the US (30). The majority of the drugs listed in Table 2 are not classified as chemotherapeutics but rather as targeted drugs, either monoclonal antibodies or small molecules, predominantly tyrosine kinase inhibitors. If these drugs are broken down according to whether they have a companion diagnostic assay linked to their use or not, there is a clear trend towards a somewhat higher response rate for the group of drugs with a companion diagnostic (4). The objective response rates for this group range from 41.0% to 80.2%, while for the group of drugs that have no companion diagnostic assay linked to their use this is from 6.8% to 45.0%. The result of this simple survey seems to indicate that integration of molecular diagnostics matters for the clinical outcome of oncology drugs.
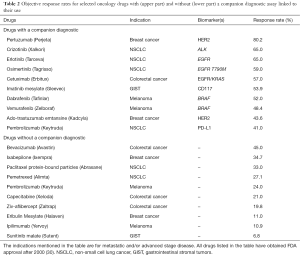
Full table
Clinical application of companion diagnostics
Another group of small molecule tyrosine kinase inhibitor that have demonstrated similar high response rates in NSCLC as the ALK inhibitors is the EGFR inhibitors, such as gefitinib (Iressa®, AstraZeneca) and erlotinib (Tarceva, Roche/Genentech). These compounds are active in NSCLC patients harboring an activating EGFR mutation (EGFR M+) and also here the objective response rates are in the range of 60% to 70% (30-32). For both gefitinib and erlotinib companion diagnostics have been developed based on a real-time PCR platform (15).
Despite strong clinical evidence that the efficacy of EGFR inhibitors is restricted to patients with EGFR M+ there still seems to lack understanding of the importance of companion diagnostic testing. An international survey, based on reply from 562 oncologists from 10 different countries, was recently presented at the European Lung Cancer Conference in Geneva, Switzerland (33). This survey showed that for nearly 25% of patients with advanced NSCLC, the EGFR M+ test result was not available at the time of treatment initiation. A significant variation between countries was observed. For Japan, this figure was only 11% while for France it was as high as 51%. Furthermore, more than half of all the oncologist surveyed stated that their treatment decision was not affected by EGFR mutation subtype. Another survey performed among medical oncologist in the USA, and presented at the ASCO meeting in 2014, showed similar results (34). So, despite having known these types of assays within oncology for a number of years, there still seem to lack understanding of the clinical importance, and the findings in these surveys demonstrate a need for further education on how critical companion diagnostic testing is.
Regulatory aspects
In the US, companion diagnostics are classified as In Vitro Diagnostic (IVD) class III products, which according to the regulations are medical devices that usually support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury (35). Furthermore, an IVD Class III product normally requires a pre-market approval (PMA) application to secure a high standard of both the analytical and clinical performance of the assays. Compared to other types of IVD submissions the PMA application requires the most comprehensive documentation level. The way that companion diagnostics are classified by the US FDA underlines the critical role of these assays in the treatment decision process for the individual patient.
The regulatory framework for companion diagnostics in the EU is the IVD Directive 98/79/EC issued in 1998 (36). However, this directive does not mention this type of assay in the definition of an IVD, and likewise, the classification system does not consider companion diagnostics at all, which of course has to do with the age of the directive. Currently, any companion diagnostics entering the EU market is classified as general IVD, which means low risk devices. Through the so-called self-certification procedure, the manufacturer performs a conformity assessment according to the IVD Directive, and then subsequently CE-mark the assay. Compared to the PMA approval process in the US, the EU self-certification procedure must be considered a very different regulatory pathway, which does not take into consideration the critical role of the companion diagnostics in relation to the treatment decision (7). However, this will be changed in the near future as the European Commission has proposed a new regulatory framework that will be more up-to-date with the current thinking when it comes to safety and effectiveness of this type of assay (19). An important change in the new regulation is that companion diagnostics will no longer be considered low risk devices, and likewise self-certification will no longer be a possibility. According to the proposed new regulation, companion diagnostics will be classified as class C, which means a high individual risk or moderate public health risk, where an erroneous result would put the patient in an imminent life-threating situation or would have major negative impact on outcome (37). Furthermore, a requirement will also be that an independent notified body should perform the conformity assessment, and that this notified body has to consult the European Medicines Agency (EMA) or one of the medical product national competent authorities (19).
Also outside the US and Europe, especially in Asia, new legislation has recently been implemented for companion diagnostic assays. In Japan, a risk based classification system similar to the one in US has been implemented, with companion diagnostics being classified as Class III products (38). However, there are some areas were the legislation differs from that of the US. One is in relation to the simultaneous regulatory approval of drug and diagnostic. In the Japanese legislation, the pharma and diagnostic companies are encouraged to cooperate from the very early stages of the drug development process in order to be able to finalize the companion diagnostic development prior to the approval of the corresponding drug. Such an approach will enable the testing laboratories to be prepared for the use of the assay in advance of the anticipated drug approval. Furthermore, the inclusion of biomarker-negative patients in the early phase clinical studies is strongly emphasized in the Japanese regulations.
According to the US FDA “List of Cleared or Approved Companion Diagnostic Devices”, 18 drugs have a companion diagnostic linked to their use so far. Beside these 18 drugs, 2 additional drugs have a complimentary diagnostic liked to their use. When the US FDA recently approved the PD-L1 checkpoint inhibitor nivolumab and atezolizumab they approved the PD-L1 IHC 28-8 pharmDx and the Ventana PD-L1 (SP142) assay as complementary diagnostics at the same time. Despite the US FDA having started to use the term, complimentary diagnostic, they have not yet officially defined this new class of diagnostic assay. However, a draft definition has recently been presented, and this definition says that a complementary diagnostic is “A test that identify a biomarker-defined subset of patients that respond particularly well to a drug and aid risk/benefit assessments for individual patients, but that are not pre-requisites for receiving the drug” (39). Table 3 lists the drugs that have been approved by the US FDA with either a companion or complementary diagnostic.
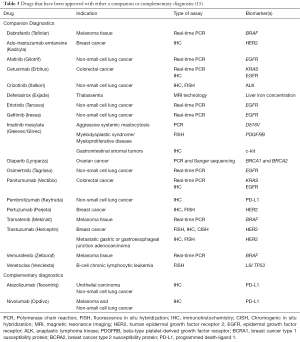
Full table
Conclusions
Drugs work at the molecular levels and this needs to be the point of reference, if we want to improve the current pharmacotherapy. For severe chronic diseases, the treatment decision should be based on solid biomarker data and not on a type of “trial and error” approach. Within the treatment of cancer, the combination of drug and diagnostic has already proved its value. Companion diagnostics have shown to be an important tool both in relation to the drug development process as well as for the treatment of the individual patients in the clinic. However, the number of companion diagnostics assays are still relatively low, but based on a recent survey among pharma companies it seems that the number will increase in the years to come, especially within oncology (40). A widespread use of this type of assay would lead to a more rational and cost-effective pharmacotherapy to the benefit of both the individual patient and the healthcare system as a whole.
Acknowledgements
The publication was supported by a grant from the Dx-Rx Institute.
Footnote
Conflicts of Interest: Jan Trøst Jørgensen has worked as a consultant for Dako, Agilent Technologies and Euro Diagnostica, and has given lectures at meetings sponsored by AstraZeneca, Merck Sharp & Dohme, and Roche. Maria Hersom has nothing to declare. All the figures included in the manuscript are original and have been developed by Dr. Hersom.
References
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med 2001;7:201-4. [Crossref] [PubMed]
- Jørgensen JT. Are we approaching the post-blockbuster era? Pharmacodiagnostics and rational drug development. Expert Rev Mol Diagn 2008;8:689-95. [Crossref] [PubMed]
- Schork NJ. Personalized medicine: Time for one-person trials. Nature 2015;520:609-11. [Crossref] [PubMed]
- Jørgensen JT. Clinical application of companion diagnostics. Trends Mol Med 2015;21:405-7. [Crossref] [PubMed]
- Carr DF, Alfirevic A, Pirmohamed M. Pharmacogenomics: Current State-of-the-Art. Genes (Basel) 2014;5:430-43. [Crossref] [PubMed]
- Olsen D, Jørgensen JT. Companion diagnostics for targeted cancer drugs - clinical and regulatory aspects. Front Oncol 2014;4:105. [Crossref] [PubMed]
- Jørgensen JT. Clinical Application of Companion Diagnostics. Clin Chem Lab Med 2016;54(10):eA254. Abstract presented at the 4th Joint EFLN – UEMS Congress, September 21-24, 2016, Warsaw, Poland. Available online: . [Accessed October 6, 2016].https://www.degruyter.com/downloadpdf/j/cclm.2016.54.issue-10/cclm-2016-0657/cclm-2016-0657.xml
- Lerner HJ, Band PR, Israel L, et al. Phase II study of tamoxifen: report of 74 patients with stage IV breast cancer. Cancer Treat Rep 1976;60:1431-5. [PubMed]
- Jørgensen JT. Companion diagnostics in oncology - current status and future aspects. Oncology 2013;85:59-68. [Crossref] [PubMed]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Jørgensen JT, Winther H. The development of the HercepTest – from bench to bedside. In: Jørgensen JT, Winther H. editor(s). Molecular Diagnostics – The Key Driver of Personalized Cancer Medicine. Pan Stanford, 2010:43-60.
- US FDA. Drug-Diagnostic Co-Development Concept Paper. Draft. April 2005. Available online: . [Accessed October 8, 2016].http://www.fda.gov/downloads/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm116689.pdf
- US FDA. List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). Updated: October 11, 2016. Available online: . [Accessed October 12, 2016].http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm
- US FDA. FDA approves new, targeted treatment for bladder cancer. FDA News Release. May 18, 2016. Available online: . [Accessed October 8, 2016].http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm501762.htm
- US FDA. Ventana PD-L1 (SP142) Assay. Premarket Approval (PMA). Available online: . [Accessed November 2, 2016].http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p160006
- Papadopoulos N, Kinzler KW, Vogelstein B. The role of companion diagnostics in the development and use of mutation-targeted cancer therapies. Nat Biotechnol 2006;24:985-95. [Crossref] [PubMed]
- US FDA. Guidance for Industry and Food and Drug Administration Staff. In Vitro Companion Diagnostic Devices. August 6, 2014. Available online: . [Accessed October 8, 2016].http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM262327.pdf
- European Commission. (2016) Proposal for a Regulation of the European Parliament and of the Council on in vitro diagnostic medical devices. 9365/3/16 REV 3. June 15, 2016. Available online: . [Accessed October 8, 2016].http://data.consilium.europa.eu/doc/document/ST-9365-2016-REV-3/en/pdf
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. LBA8_PR - KEYNOTE-024: Pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) as first-line therapy for advanced NSCLC with a PD-L1 tumor proportion score (TPS) ≥50%. Abstract presented at ESMO 2016 Congress, October 7 - 11, 2016, Copenhagen, Denmark. Available online: . [Accessed October 12, 2016].https://cslide.ctimeetingtech.com/library/esmo/browse/itinerary/5286/2016-10-09#2z94T0v3
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Socinski M, Creelan B, Horn L, et al. LBA7_PR - CheckMate 026: A phase 3 trial of nivolumab vs investigator's choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage iv/recurrent programmed death ligand 1 (PD-L1) − positive NSCLC. Abstract presented at ESMO 2016 Congress, October 7 - 11, 2016, Copenhagen, Denmark. Available online: . [Accessed October 12, 2016].https://cslide.ctimeetingtech.com/library/esmo/browse/itinerary/5286/2016-10-09#2z94T0v3
- Jørgensen JT. Companion diagnostic assays for PD-1/PD-L1 checkpoint inhibitors in NSCLC. Expert Rev Mol Diagn 2016;16:131-3. [Crossref] [PubMed]
- Daud AI, Wolchok JD, Robert C, et al. Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J Clin Oncol 2016;34:4102-9. [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Jørgensen JT. The importance of predictive biomarkers in oncology drug development. Expert Rev Mol Diagn 2016;16:807-9. [Crossref] [PubMed]
- US FDA. FDA expands use of Xalkori to treat rare form of advanced non-small cell lung cancer. FDA News Release, March 11, 2016. Available online: . [Accessed October 21, 2016].http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm490329.htm
- US. Prescribing Information for Xalkori (crizotinib) capsules, for oral use. Revised 3/2016. Available online: . [Accessed October 21, 2016].http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202570s016lbl.pdf
- Shaw A, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- US FDA. Drugs@FDA. Available online: . [Accessed October 21, 2016].http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Spicer J, Tischer B, Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: Global trends and differences. Annals of Oncology 2015;26 Supplement 1:i57-i61. [Crossref]
- Green MR, Willey J, Buettner A, et al. Molecular testing prior to first-line therapy in patients with stage IV nonsquamous non-small cell lung cancer (NSCLC): A survey of U.S. medical oncologists. J Clin Oncol 32:5s, 2014 (suppl; abstr 8097).
- US FDA. Guidance for Industry and FDA Staff. In Vitro Diagnostic (IVD) Device Studies - Frequently Asked Questions. June 25, 2010. Available online: . [Accessed October 25, 2016].http://www.fda.gov/downloads/MedicalDevices/.../ucm071230.pdf
- European Parliament. Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Available online: . [Accessed October 25, 2016].http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1998:331:0001:0037:EN:PDF
- Pignatti F, Ehmann F, Hemmings R, et al. Cancer drug development and the evolving regulatory framework for companion diagnostics in the European union. Clin Cancer Res 2014;20:1458-68. [Crossref] [PubMed]
- Nagai S, Urata M, Sato H, et al. Evolving Japanese regulations on companion diagnostics. Nat Biotechnol 2016;34:141-4. [Crossref] [PubMed]
- Jørgensen JT. Companion and Complementary Diagnostics – Clinical and Regulatory Perspectives. Trends Cancer 2016;2:706-12. [Crossref]
- Milne CP, Cohen JP, Chakravarthy R. Market watch: Where is personalized medicine in industry heading? Nat Rev Drug Discov 2015;14:812-3. [Crossref] [PubMed]


