Effects of curcumin on Helicobacter pylori infection
Introduction
Helicobacter pylori (H. pylori) is a Gram-negative bacterium that selectively colonizes the human gastric epithelium and is epidemiologically linked to stomach and colorectal cancer (1). In addition, it is implicated in the etiology of gastritis and peptic ulcers. Antibody therapy coupled with other treatments is highly effective, but not without complications (2). Over 50% of the world population is infected with these bacteria. The resistance of these bacteria to common antibiotics has been related to the genetic variability and to its ability to develop biofilm (3). In addition, Helicobacter infection has been connected with development of allergies (4).
Lately, the focus of numerous investigations has switched to various herbal agents shown to have significant antibacterial activity against H. pylori (5). One of these agents is curcumin (6,7). In addition, curcumin also serves as a biofilm-disrupting agent (8), suggesting multiple roles of curcumin in inhibition of H. pylori infection.
Curcumin, commonly known as turmeric, is usually a mixture of three curcumoids (curcumin, demethocycurcumin, and bisdemethoxycurcumin) and volatile oil (9). Numerous studies have reported that curcumin has a wide range of biological activities including antimicrobial, antioxidant, antitumor (10), and anti-inflammatory effects. In addition, curcumin has some immunosuppressive activities (11) including expression of cytokines such as IL-1 and TNF-α (12,13). On the other hand, curcumin enhanced phagocytic activity of macrophages (14). In our study, we compared the antibacterial effects of five different types of curcumin.
Methods
Animals
Female, 8-week-old BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). All animal work was done according to the University of Louisville IACUC protocol. Animals were sacrificed by CO2 asphyxiation.
Samples
Curcumin C3 complex 95% (sample #1) was purchased from Sabinsa (Sabinsa Corp., East Windsor, NJ, USA), curcumin powder 65% (sample #2) and curcumin 94% (sample #3) from Sigma (St. Louis, MO, USA), curcumin 95 (95%, sample #4) from Jarrow Formulas (Los Angeles, CA, USA), and curcumin 95% (sample #5) from Orcas Naturals (Landing, NJ, USA).
Bacteria
H. pylori strain ATCC43504 was purchased from ATCC (American Type Culture Collection, Manassas, VA, USA) and cultured on brain-heart infusion (BHI) agar (Sigma) supplemented with 7% sheep blood and incubated at 37 °C under microaerobic conditions.
Lipid peroxide (LPO) level and myeloperoxidase (MPO) activity
Gastric mucosal tissues were scrapped and homogenized in 10 mmol/L Tris buffer (pH 7.4). LPO levels were measured as described by Ohkawa et al. (15). MPO activity was determined by the modified method of Krawisz et al. (16).
Urease activity
Urease activity in the homogenized gastric tissue was performed as described by O’Riordan et al. (17).
Enumeration of colonized bacteria
Stomach samples were homogenized in phosphate buffer saline (PBS), cultured on the brucella agar plates incubated under microaerobic conditions. Five days after cultivation, colony counts were performed (18).
Anti-H. pylori antibodies
Serum anti-H. pylori IgG were measured using an enzyme-linked immunosorbent assay (ELISA). Isolates of H. pylori were used as an antigen at 25 µg protein/well. After incubation and washing, 100 mg of serum was added. Reaction was followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma, St. Louis, MO, USA). Optical density was measured using a STL ELISA reader (Tecan U.S., Research Triangle Park, NC) at 405 nm.
Biofilm formation
Bacteria were grown in glass tubes. BHI broths supplemented with 2% β-cyclodextrin (BCD) and 0.016% dimethyl sulfoxide (DMSO) were incorporated as blank and control, respectively. After 7 days of incubation, all culture medium was removed. The test tubes were washed twice with PBS, dried for 30 min at 60 °C and 10 mL of 0.1% crystal violet (Sigma) was added for 5 minutes. Unbound stain was discarded and the tubes were again dried for 30 min at 60 °C. Bound crystal violet was decolorized with ethanol/acetone mixture (80:20, v/v). The level of biofilm formation was quantified by measuring the absorbance of the solution at 570 nm using a spectrophotometer (19).
ELISA
Serum levels of IFN-γ, IL-4, gastrin, and somatostatin were determined using an ELISA assay as described by Zhang et al. (20). Anti-IFN-γ and IL-4 Quantikine ELISA kits were purchased from B&D Systems (Minneapolis, MN, USA), anti-somatostatin ELISA kit was purchased from LSBio (Seattle, WA, USA), and anti-gastrin ELISA kit from Sigma. All kits were used according to manufacturer’s instruction.
Minimum inhibitory concentration test
Technique using Mueller-Hinton agar (Oxoid, UK) described by Pattiyathanee et al. (19) was used.
Statistics
Student t-test was used to statistically analyze the data.
Results
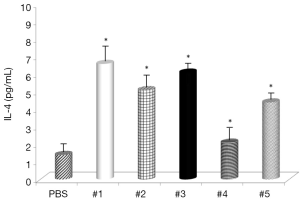
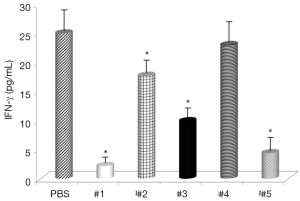
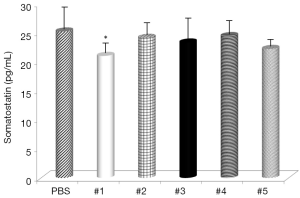
Evaluation of the changes in IL-4 and IFN-γ levels showed that all samples significantly increased IL-4 serum levels (Figure 1), with the highest effects with samples #1 and #3 and all samples, with the exception of #4, significantly decreased IFN-γ levels (Figure 2). Somatostatin levels significantly lowered only sample #1 (Figure 3); and gastrin levels showed significant effects only with samples #1 and #5 (Figure 4).

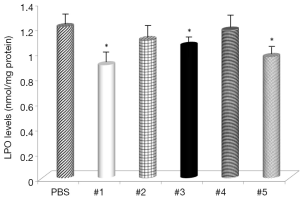
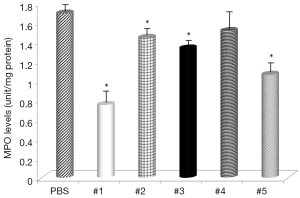
Gastric levels of LPO were significantly decreased by samples #1, #3, and #5 (Figure 5) and all samples, except sample #4, had decreased levels of MPO (Figure 6). The negative control mice levels (without infection) were 0.91 nmol/mg protein with LPO, and 0.49 units/mg protein with MPO activity.
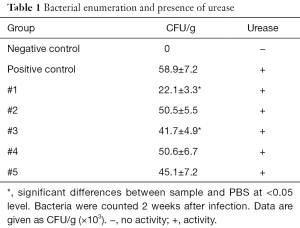
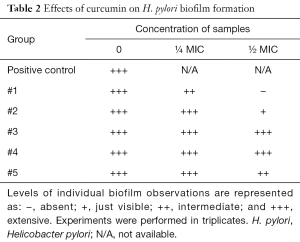
The next part of the study was focused on bacterial enumeration and presence of urease. Urease was detected in all tested samples, but samples #1 and #3 significantly lowered the H. pylori counts (Table 1). We then studied the effects of tested samples on H. pylori formation. As summarized in Table 2, in higher concentration, we found strong effects of samples #1, #2, and #5 and in lower concentration, only sample #1 showed small effects.
Full table
Full table
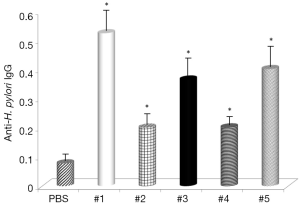
The final part of this study measured direct effects of curcumin supplementation of levels of anti-H. pylori IgG. Results given in Figure 7 show that all samples increased production of specific antibodies, with samples #1, #3 and #5 showing the strongest effects.
Conclusions
H. pylori is a highly mobile Gram-negative bacterium, selectively colonizing in the human stomach. This colonization is etiologically associated with gastritis and peptic ulcers. In addition, an increased risk of gastric adenocarcinoma has been well established (21). With over 50% of the population (higher in developing countries) infected with H. pylori, various drugs have been routinely used for eradication of this infection. However, steadily increasing resistance to antibiotics, undesirable side effects, and raising costs have given rise to the recent surge of interest in alternative approaches (22,23).
Curcumin, the principle yellow pigment from the rhizome of turmeric (Curcuma longa), is known for a wide range of biological and pharmaceutical effects, most of all as an anti-inflammatory agent (24). Not surprisingly, curcumin has been evaluated as a potential anti-H. pylori agent. Curcumin supplementation was found to significantly downregulate MMP3 and MMP9 activities (25). A mouse study showed that orally-given curcumin caused significant inhibition of gastric inflammation induced by H. pylori infection (26).
With significant effects of curcumin on H. pylori infection, the aim of this study was to directly compare the antibacterial effects of five different types of curcumin to see if these effects are dependent on the individual type of curcumin. For our study we used samples with already well-established anti-inflammatory effects (27).
To better explore the mechanisms of curcumin effects on this infection, IFN-γ, IL-4, and somatostatin represent good molecules for this purpose, as it is known that IFN-γ levels are elevated by H. pylori infection (28). IL-4 is an anti-inflammatory cytokine, unknown to be depressed by H. pylori (29) and somatostatin is a regulatory peptide needed for IL-4 mediated resolution of H. pylori-related gastritis (30). Gastrin is secreted by G cells from the pyloric antrum and is involved in the stimulation of gastric acid formation and release. As its levels are depressed as a result of H. pylori infection, we can speculate that the control of gastrin levels might result in modulation of gastritis. Significant effects of curcumin on all four molecules strongly support the hypothesis that curcumin can reduce effects of H. pylori infection.
The levels of LPO, an oxidative damage index, were decreased by supplementation with curcumin and in some cases almost reached the levels found in non-infected animals. These results suggested that curcumin treatment inhibited the H. pylori-induced increase in LPO abundance in the gastric mucosa and that curcumin does have antioxidant effects. LPO is considered to be a marker of oxidative membrane damage (31). Review of MPO activity indicated that curcumin addition attenuated neutrophil infiltration in the gastric mucosa, similar to the study using Angelica keiskei (32).
The next phase of our study focused directly on bacteria and their action. Enumeration of bacterial cells in the infected animal stomach showed that curcumin supplementation reduced the total amount of H. pylori bacteria. Urease-positive bacteria form thick biofilms in the stomach of the host (33). Curcumin was able to inhibit the growth of H. pylori at a MIC value of 18 µg/mL, which corresponded with the findings of others (19). When used at sub-inhibitory levels (½ and ¼ MIC), the effects on biofilm were observed.
Next, then analyzed the effects of curcumin supplementation on formation of anti-H. pylori IgG antibodies. The results showed that the same samples which reduced the number of bacteria also increased the formation of specific antibodies, supporting the hypothesis that curcumin has strong immunostimulating properties.
In summary, we directly compared the effects of five various samples of curcumin on H. pylori-induced gastritis. Similar to a previous study on inflammatory effects of curcumin (Vetvicka and Vetvickova, 2016), sample #1 (Sabinsa) was the most active of all samples in all of our tests, followed by samples #5 (Orcas Natural) and #3 (highly purified curcumin from Sigma). The two remaining samples were significantly less active, showing that despite the clear activity of curcumin in general, not every commercial sample of curcumin is highly active.
Acknowledgements
Funding: Department of Pathology funds.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All animal work was done according to the University of Louisville IACUC protocol.
References
- Parsonnet J. Bacterial infection as a cause of cancer. Environ Health Perspect 1995;103 Suppl 8:263-8. [Crossref] [PubMed]
- Graham DY, Lew GM, Malaty HM, et al. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology 1992;102:493-6. [Crossref] [PubMed]
- García A, Salas-Jara MJ, Herrera C, et al. Biofilm and Helicobacter pylori: from environment to human host. World J Gastroenterol 2014;20:5632-8. [Crossref] [PubMed]
- Richter J, Vetvicka V, Kral V, et al. Helicobacter infection and allergy in majority and Roma population in the Czech Republic. Am J Immunol 2014;10:166-72. [Crossref]
- Zaidi SF, Muhammad JS, Usmanghani K, et al. Review: Pharmacological ins and outs of medicinal plants against Helicobacter pylori: A review. Pak J Pharm Sci 2015;28:1171-6. [PubMed]
- Mahady GB, Pendland SL, Yun G, et al. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res 2002;22:4179-81. [PubMed]
- Thong-Ngam D, Chatsuwan T. Antibacterial activity of Aloe vera, curcumin, and plau-noi against Helicobacter pylori. Thai J Gastroenterol 2007;8:5-11.
- Hegarty JP, Dowd MT, Baker KH. Occurrence of Helicobacter pylori in surface water in the United States. J Appl Microbiol 1999;87:697-701. [Crossref] [PubMed]
- Hamidpour R, Hamidpour S, Hamidpour M, et al. Turmeric (Curcuma longa): from a variety of traditional medicinal application to its novel roles as active antioxidant, anti-inflammatory, anti-cancer, and anti-diabetes. Int J Pharmacol Phytochem Ethnomed 2015;1:37-45.
- Ruby AJ, Kuttan G, Babu KD, et al. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett 1995;94:79-83. [Crossref] [PubMed]
- Gao X, Kuo J, Jiang H, et al. Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol 2004;68:51-61. [Crossref] [PubMed]
- Chan MM. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem Pharmacol 1995;49:1551-6. [Crossref] [PubMed]
- Moon DO, Jin CY, Lee JD, et al. Curcumin decreases binding of Shiga-like toxin-1B on human intestinal epithelial cell line HT29 stimulated with TNF-alpha and IL-1beta: suppression of p38, JNK and NF-kappaB p65 as potential targets. Biol Pharm Bull 2006;29:1470-5. [Crossref] [PubMed]
- Bisht K, Choi WH, Park SY, et al. Curcumin enhances non-inflammatory phagocytic activity of RAW264.7 cells. Biochem Biophys Res Commun 2009;379:632-6. [Crossref] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8. [Crossref] [PubMed]
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984;87:1344-50. [PubMed]
- O'Riordan AA, Morales VA, Mulligan L, et al. Alkyl hydroperoxide reductase: a candidate Helicobacter pylori vaccine. Vaccine 2012;30:3876-84. [Crossref] [PubMed]
- Attaran B, Falsafi T, Moghaddam AN. Study of biofilm formation in C57Bl/6J mice by clinical isolates of Helicobacter pylori. Saudi J Gastroenterol 2016;22:161-8. [PubMed]
- Pattiyathanee P, Vilaichone R, Chaichanawongsaroj N. Effect of curcumin on Helicobacter pylori biofilm formation. Afr J Biotechnol 2009;8:5106-15.
- Zhang S, Mo F, Luo Z, et al. Flavonoid glycosides of polygonum capitatum protect against inflammation associated with Helicobacter pylori infection. PLoS One 2015;10:e0126584. [Crossref] [PubMed]
- Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol 2006;1:63-96. [Crossref] [PubMed]
- Kim TS, Shin K, Jeon JH, et al. Comparative analysis of anti-Helicobacter pylori activities of FEMY-R7 composed of Laminaria japonica and Oenothera biennis extracts in mice and humans. Lab Anim Res 2015;31:7-12. [Crossref] [PubMed]
- Lee SY, Shin YW, Hahm KB. Phytoceuticals: mighty but ignored weapons against Helicobacter pylori infection. J Dig Dis 2008;9:129-39. [Crossref] [PubMed]
- Guimarães MR, Leite FR, Spolidorio LC, et al. Curcumin abrogates LPS-induced pro-inflammatory cytokines in RAW 264.7 macrophages. Evidence for novel mechanisms involving SOCS-1, -3 and p38 MAPK. Arch Oral Biol 2013;58:1309-17. [Crossref] [PubMed]
- Kundu P, De R, Pal I, et al. Curcumin alleviates matrix metalloproteinase-3 and -9 activities during eradication of Helicobacter pylori infection in cultured cells and mice. PLoS One 2011;6:e16306. [Crossref] [PubMed]
- Santos AM, Lopes T, Oleastro M, et al. Curcumin inhibits gastric inflammation induced by Helicobacter pylori infection in a mouse model. Nutrients 2015;7:306-20. [Crossref] [PubMed]
- Vetvicka V, Vetvickova J. Strong anti-inflammatory effects of curcumin. J Nutr Health Sci 2016;3:205.
- Wang YC, Chen CL, Sheu BS, et al. Helicobacter pylori infection activates Src homology-2 domain-containing phosphatase 2 to suppress IFN-γ signaling. J Immunol 2014;193:4149-58. [Crossref] [PubMed]
- Karttunen R, Karttunen T, Ekre HP, et al. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut 1995;36:341-5. [Crossref] [PubMed]
- Kao JY, Pierzchala A, Rathinavelu S, et al. Somatostatin inhibits dendritic cell responsiveness to Helicobacter pylori. Regul Pept 2006;134:23-9. [Crossref] [PubMed]
- Craig PM, Territo MC, Karnes WE, et al. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut 1992;33:1020-3. [Crossref] [PubMed]
- Kim A, Lim JW, Kim H, et al. Supplementation with Angelica keiskei inhibits expression of inflammatory mediators in the gastric mucosa of Helicobacter pylori-infected mice. Nutr Res 2016;36:488-97. [Crossref] [PubMed]
- Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161-81. [Crossref] [PubMed]


