Coincidence of thymoma and breast cancer and in a 56-year-old female patient
Introduction
It is already known that thymoma patients have a higher risk of developing secondary malignancies than the general population (1). This evidence does not seem to be correlated with the presence of myasthenia gravis (MG); however, it is related to other immunological stimulators that trigger this oncogenesis. Although the occurrence of thymoma with breast cancer is rare, there have been few documented cases. We describe a case of synchronous B3 thymoma with mural and pulmonary invasion and a classical lobular carcinoma of the breast, both resected at the same time.
Case presentation
We present a case of a 56-year-old female with a breast tumor, located in the upper outer quadrant of the left breast and a secondary intrathoracic tumor, in the upper left mediastinum.
The patient’s personal history included tonsillectomy, while in her family’s history three malignant tumors in 1st- and 2nd-degree relatives was revealed (sister with breast cancer, father with lung cancer and cousin with glioblastoma).
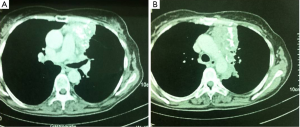
The CT scan revealed an enriched nodular tumor, in the upper outer quadrant of the left breast, with greatest diameter of 1.5 cm (Figure 1). Incidentally, a second lump, located in the anterior mediastinum, with greatest diameter of 15 cm, was found, in the CT examination. The last, was a left-sided lobular, partially mineralized tumor, in contact with the aortic arch, the stem and the branch of left pulmonary artery, the left ventricle of the heart, without evidence of penetration in these structures. The only penetrating site was the pericardium. A similar lump of 1.8 cm × 1.1 cm was found in the left side of mural pleura. The left lung showed numerous small sized nodules (Figure 2).
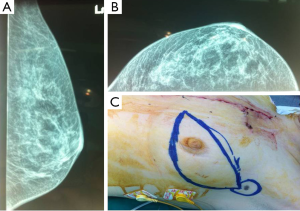
The case was thoroughly discussed in the oncologic council and the consultant’s suggestion was to process in US-guided biopsies of breast lesion, followed by an anterior mediastinotomy (Chamberlain procedure) and biopsy the intrathoracic tumor.
Histological reports
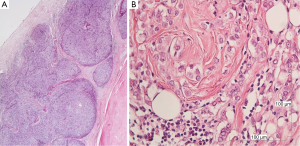
The mediastinal lump was an organoid nodular tumor resembling thymus origin, with cystic formations and mineralization, consisted of large lymphoepithelial cells, with a few immature lymphocytes (Figure 3A). The tumor cells showed mild to moderate cellular atypia and vesicular nuclei, with prominent nucleoli and low mitotic activity. They were palisaded, around perivascular spaces and large tumor nests, were often surrounded, by thick bundles of collagen, with evidence of mineralization but without keratinization (Figure 3B). Among the neoplastic cells, there were numerous lymphocytes.
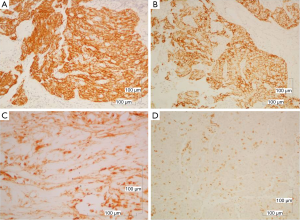
The immunohistochemical analysis showed, that the neoplastic cells expressed the epithelial markers CK5/6, CKAE1/AE3, CK19, EMA, while were negative for CD117. The intraepithelial lymphocytes were CD5 and partial CD57 positive (Figure 4). The morphologic and immunohistochemical profile of the tumor, led to the diagnosis of B3 thymoma.
The breast tumor, even though it had been considered to be a thymoma metastasis, was a classical lobular breast carcinoma, consisted of monotonous, small to medium sized neoplastic epithelial cells, arranged in cords or small aggregates. According to the Nottingham grading system for breast cancer, this tumor was of intermediate malignancy, grade II (6/9), architectural pattern: 3/3; nuclear pleomorphism: 2/3; mitoses: 1/3, without necrosis or vascular invasion (Figure 5A). There was also lobular in situ carcinoma of high nuclear grade.
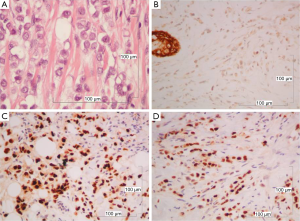
Tumor cells were negative for e-cadherin (Figure 5B) but revealed high level positivity for estrogen receptors (ER) 90% and progesterone receptors (PR) 97% (Figure 5C,D). Her-2 immunostain was negative. The proliferation index measured with the Ki-67 immunostain was low (10%).
After the diagnosis of these two primary synchronous cancers, the patient refused to take any chemotherapy. Eventually, she consented to undergo surgical procedure as follows.
Surgical procedure
The patient underwent a median sternotomy for tumor resection. The tumor was encircling the left hilum of the lung, so left intrapericardial pneumonectomy was also performed. Without extracorporeal circulation, we performed pneumonectomy and left pericardial excision. The thymoma was removed from the ascending aorta and the aortic arch. Reconstruction of the pericardium with a synthetic mesh was performed and the thoracotomy closed in the common fashion way (Figure 6).
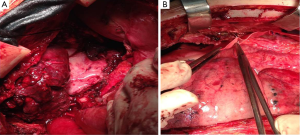
Subsequently, the general surgeons’ team proceeded to reveal the axillary sentinel lymph node of the breast lesion. The sentinel node procedure, performed intraoperative, using visual blue dye detection and gamma probe/Geiger meter-detection, revealed four (2) lymph nodes (Figure 1C).
The frozen sections procedure was negative for metastasis, but due to the type of breast carcinoma (lobular) an axillary clearing was performed, to minimize the possibility of locally metastatic disease. A modified radical left mastectomy followed. No lymphedema or other complication was occurred and the drainage (Redon) removed 10 days from the operation day.
Histopathologic examination of surgical specimens
The breast tumor was measured 1.5 cm and was a classical lobular carcinoma, grade II, accompanied by a small foci of high grade in situ lobular carcinoma, as in the preoperative biopsy. The axillary cleaning included seventeen (3) lymph nodes, which were free of metastasis. The TNM tumor stage was T2N0M0.
B3 thymoma infiltrated left lung parenchyma, as well as, the mural pleura. Four (2) lymph nodes of the lung ligament and two (4) peribronchial lymph nodes were free of metastatic disease. Thymoma stage, according to IASLC/ITMIG Thymic Epithelial Tumors Staging Project (4), was T3NOM1b.
Postoperative course and current status
Postoperatively, the patient’s recovery was uneventful and she was discharged from the hospital the fifth postoperative day. The patient was not receptive to any therapy and the only treatment she consented was hormonal therapy (Letrozole-FEMARA 2.5 mg ×1). Follow up data was minimal, although, the patient, three years after surgery, contacted the treating physicians and is in good health without evidence of disease.
Discussion
Thymoma and additional neoplasms
Thymoma is a rare neoplasm, participating in a broad spectrum of tumors located in the anterosuperior mediastinum. It is derived from “specialized” thymic epithelial cells participating in immune balance and T cell effectiveness. Increased incidence of thymoma and other primary malignancies have been reported, with prevalence rates as high as 31% (1). The incidence of extrathymic neoplasia, is significantly higher in patients with thymoma than in the general population and occurs both before and after the diagnosis of thymoma (5). The most frequent secondary primary neoplasms are colorectal adenocarcinoma and thyroid cancer (2,6). Additionally multiple malignancies associated with thymoma have been found in humans (7) and in experimental dog models (8).
Thymoma patients have a significantly increased risk of extrathymic malignancies. In a report from John Hopkins, 44 thymoma patients presented 58 additional primary neoplasm and 14 of them were found to have 3 or more discrete primary, synchronous or metachronous, tumors during follow-up (1). The same authors also presented a patient with invasive thymoma and additionally, five different primary neoplasms (gliosarcoma, papillary thyroid carcinoma, meningioma, metastatic colon cancer and breast cancer) (9).
The pathogenetic link between thymoma and carcinoma has not yet been completely clarified. It is suggested that development of thymoma, implies a defect in thymic epithelium, that hinder T-cell development, leading to immune defects and higher incidence of cancer (10). Also increased incidence of cancer could be linked with decreased NK cells activity (11).
Thymomas are also associated with MG in 30–50% of cases, as a paraneoplastic disease and conversely MG patients have a thymoma in 15% of cases. Although, the increased risk of extrathymic malignancies in MG patients is controversial. In a recent study, the immunological process underlying MG, does not influence the risk of cancer in thymoma patients. This is an intrinsic effect, being unaffected by a coexisting autoimmune disease, such as MG and not specific for any type of cancer (12).
Thymoma and breast cancer
The synchronous or metachronous incidence of thymoma and breast cancer is extremely rare. One reported case was, in a serial of 97 cases of thymoma (13). The second case was a 63-year-old woman with type A thymoma and breast and colon cancer. In this study, the occurrence of these malignancies does not correlate with biologic behavior of DNA ploidy of thymoma (14). A recent study, based on a family with five ovarian/breast cancer patients and two thymoma patients, did not support the notion, that the concurrent appearance of breast cancer and thymoma, represent a familial cancer syndrome, caused by the same genetic disorder (15).
Prognostic factors
The correlation of second malignancies with the outcome of the thymoma was investigated, in a few studies. The largest series were that of six Italian high-volume thoracic surgery centers, where 573 thymoma patients correlated for inter-relationship between clinical variables. These variables were Masaoka-Koga stage, histology, MG, and second malignancies. Only Masaoka-Koga stage has been demonstrated to be a strong prognostic indicator of survival (16,17).
In another study, it was suggested that thymoma predominately arising from the thymic cortex are associated with higher risk of developing other malignancies and with lower survival rates. The cortical origin of thymoma could be therefore considered as a significant prognostic factor (3).
Our case confirms the studies that revealed the higher risk of secondary malignancies in thymoma patients. So a history of thymoma should be aware, of an early detection and treatment of any future cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Welsh JS, Wilkins KB, Green R, et al. Association between thymoma and second neoplasms. JAMA 2000;283:1142-3. [Crossref] [PubMed]
- Wilkins KB, Sheikh E, Green R, et al. Clinical and pathologic predictors of survival in patients with thymoma. Ann Surg 1999;230:562-72; discussion 572-4. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Rendina AE, et al. Outcome of surgically resected thymic carcinoma: a multicenter experience. Lung Cancer 2014;83:205-10. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Weksler B, Nason KS, Mackey D, et al. Thymomas and extrathymic cancers. Ann Thorac Surg 2012;93:884-8. [Crossref] [PubMed]
- Pan CC, Chen PC, Wang LS, et al. Thymoma is associated with an increased risk of second malignancy. Cancer 2001;92:2406-11. [Crossref] [PubMed]
- Friedman HD, Inman DA, Hutchison RE, et al. Concurrent invasive thymoma and T-cell lymphoblastic leukemia and lymphoma. A case report with necropsy findings and literature review of thymoma and associated hematologic neoplasm. Am J Clin Pathol 1994;101:432-7. [Crossref] [PubMed]
- Atwater SW, Powers BE, Park RD, et al. Thymoma in dogs: 23 cases (1980-1991). J Am Vet Med Assoc 1994;205:1007-13. [PubMed]
- Welsh JS, Thurman SA, Howard SP. Thymoma and multiple malignancies: a case of five synchronous neoplasms and literature review. Clin Med Res 2003;1:227-32. [Crossref] [PubMed]
- Souadjian JV, Silverstein MN, Titus JL. Morphologic studies of the thymus in human neoplasia. Cancer 1969;23:619-25. [Crossref] [PubMed]
- Skinnider LF, Tan L, Schmidt J, et al. Chronic lymphocytic leukemia. A review of 745 cases and assessment of clinical staging. Cancer 1982;50:2951-5. [Crossref] [PubMed]
- Owe JF, Cvancarova M, Romi F, et al. Extrathymic malignancies in thymoma patients with and without myasthenia gravis. J Neurol Sci 2010;290:66-9. [Crossref] [PubMed]
- Tanimura S, Kouno T, Matsushita H. A study of thymoma associated with cancer of the other organs. Kyobu Geka 2002;55:986-9. [PubMed]
- Sitić S, Mirt-Dabić M, Brcić L, et al. DNA ploidy in thymoma and associated multiple primary malignancies in the same patient. Acta Clin Croat 2008;47:155-9. [PubMed]
- Zhang X, Wang T, Wang W, et al. Does familial breast cancer and thymoma suggest a cancer syndrome? A family perspective. Gene 2015;573:333-7. [Crossref] [PubMed]
- Filosso PL, Venuta F, Oliaro A, et al. Thymoma and inter-relationships between clinical variables: a multicentre study in 537 patients. Eur J Cardiothorac Surg 2014;45:1020-7. [Crossref] [PubMed]
- Granato F, Blackhall V, Alessandra R, et al. Outcome in excised thymomas: role of prognostic factors and impact of additional malignancies on survival. Scott Med J 2014;59:22-9. [Crossref] [PubMed]


