Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and Ebola
Introduction
For almost two decades, leading scientists and health officials have warned that we must prepare for a potentially devastating global pandemic of an infectious disease. Initial concern was focused on the avian influenza A (H5N1) virus. More recently, people in West Africa have endured a devastating outbreak of Ebola virus disease. Several other emerging viruses are believed to seriously threaten global health and global security. To prepare, scientists have been urged to discover new vaccines and treatments for these emerging viruses. At the same time, political leaders have been urged by global health experts to invest millions in a “top down” restructuring of the global health system.
This article takes a different view. It focuses on an alternative approach to the scientific discovery of treatments for individual patients, reviews the mechanisms of action and clinical experience with specific drugs that might be useful, and considers whether or not recent lessons regarding this “bottom up” approach to treatment have been learned.
Reductionist science and the discovery of new treatments
In a recent report, van Vught and colleagues sought to explain why hospitalized patients are susceptible to nosocomial pneumonia (1). They assumed that one reason might be immune suppression caused by the conditions for which they had been hospitalized. To address this question, they compared the systemic host responses of ICU patients with hospital-acquired and community-acquired pneumonia (HAP and CAP, respectively). They measured 19 plasma biomarkers and determined genome-wide gene expression profiles for the two groups. To their surprise, they found that levels of inflammatory cytokines and endothelial and coagulation responses in the two groups were similar, as were most measures of clinical outcome. Although some of the biomarker responses in HAP patients were “modestly mitigated” (under-expression of type 1 interferon and elevated expression of “cell junction and mobility gene signatures”), subgroup analyses, including the presence or absence of COPD and the type of infection, confirmed the overall findings (1). Not surprisingly, HAP patients were admitted to the ICU later (mean 8 days) than CAP patients (mean 0 days), but mortality rates in the two groups were not statistically different. Although overall clinical outcomes were similar in HAP and CAP patients, no information was provided on whether host responses were different in those who survived compared with those who died.
The study by van Vught et al. adds to our understanding of the many mechanisms involved in the host response to infection. Like most descriptive studies, it provides scientific explanations for what happens, yet offers no guidance on how to improve outcomes in patients with pneumonia, sepsis and other forms of critical illness. More than 100 clinical trials have been undertaken to test drugs designed to modify individual signaling molecules or pathways involved in the host response to sepsis (2). None of these trials has led to meaningful improvement in patient survival. Suggestions to improve the research model have included (I) identifying plausible targets for intervention; (II) improving patient selection, stratification and staging; (III) developing new designs for early phase studies; and (IV) creating new large-scale collaborative groups of investigators (2). Nonetheless, plausible targets for intervention have almost always been chosen based on findings in animal models that focused on individual molecules (e.g., TLR4), pathways (e.g., coagulation) or systems (e.g., innate immunity) known to be associated with adverse outcomes. These choices reflect an approach to discovery that seeks incremental improvements in scientific understanding. It is epitomized most recently by systems and computational biology. The study by van Vught et al. is a good example of this reductionist approach to discovery.
A different approach to discovering new treatments
A different approach to therapeutic discovery moves in the opposite direction. It starts with the recognition that a beneficial clinical phenotype has already been observed with treatment, and it then uses the tools of reductionist science to investigate the mechanism(s) that brought this about. It is important to understand that although this alternative is informed by the discoveries of reductionist science, it is driven primarily by an unwavering focus on the clinical (phenotypic) benefits that have been (or seem to have been) achieved with treatment. In effect, it exemplifies a Darwinian approach to therapeutic discovery.
A good example of this approach is the propensity-matched observational studies of Mortensen and colleagues (3,4). They studied patients hospitalized with community-acquired pneumonia who had been treated with statins and angiotensin receptor blockers (ARBs). In their initial study (3), outpatient treatment with either a statin or an ARB prior to hospitalization was associated with an approximately 30% reduction in 30-day all-cause mortality, and inpatient treatment with either drug was similarly or more effective (Table 1). In a more recent study using slightly different methodology, outpatient treatment with a combination of the two drugs was 60% effective in reducing 30-day all-cause mortality (Table 1) (4).

Full table
A large body of earlier experimental and clinical research had suggested that statins and ARBs might be effective in treating pneumonia patients. These drugs had been developed by cardiovascular scientists to treat patients with heart disease (statins) and hypertension (ARBs). Gradually, investigators realized they also had broad anti-inflammatory and immunomodulatory (pleiotropic) activities that affected, among other things, inflammatory and anti-inflammatory cytokines and chemokines, the complement cascade, coagulation factors, oxidative stress, macrophage and T cell polarization, late mediators of inflammation [e.g., high mobility group box 1 (HMGB1), specialized pro-resolving mediators of inflammation (e.g., lipoxins, resolvins), mitochondrial biogenesis and energy metabolism (5,6). These factors are now acknowledged to actively affect the host response to sepsis (7), pneumonia (8), and other forms of acute critical illness. Furthermore, cardiovascular investigators have published more than 80 reports showing that combination statin/ARB treatment is more effective than treatment with either agent by itself (9,10; DS Fedson, unpublished observations).
A full discussion of the many drugs (including PPAR and AMPK agonists) that might be useful in modifying the host response to critical illness, and the mechanisms by which they might act, is beyond the scope of this article. Instead, the focus will be on treatment with statins and ARBs and how they affect the host response.
Endothelial and epithelial dysfunction in critical illness
There is growing recognition that endothelial dysfunction and the loss of endothelial barrier integrity are central to the pathophysiology of bacterial sepsis and acute lung injury (7,11,12). Many systemic virus diseases (13), including influenza (14), dengue and Hantavirus pulmonary syndrome (15), are also characterized by endothelial dysfunction. Figure 1 shows the vascular endothelium in its resting state (on the left) and many of the changes in endothelial cell function that occur with sepsis (on the right) (7). The disruption of tight junctions between endothelial cells leads to a loss of barrier integrity, followed by the leak of fluid from the blood into interstitial tissues and beyond (e.g., the alveoli in pneumonia). Inflammatory changes facilitate the recruitment of macrophages and neutrophils that adhere to and transition through the endothelium. These and other changes activate the coagulation cascade, which in turn further stimulates inflammation and often establishes a feed-forward cycle in which more inflammation causes even more endothelial injury. Some of the signaling molecules involved in maintaining endothelial barrier integrity and in its disruption are shown in Figure 1 (7). Others that play important roles in endothelial cell signaling include the angiopoietin (Angpt)/Tie2 signaling axis, angiotensin-converting enzyme 2 (ACE2), vascular endothelial cadherin (VE-cadherin), claudins, C3a/C5a, RhoA/Rac1 GTPases, matrix metalloproteinases (MMPs), and sphingosine-1-phosphate-1 (S1P1) (7,8,11,12). Many other facets of endothelial activity are also involved, including redox metabolism (16) and mitochondrial function (17,18).
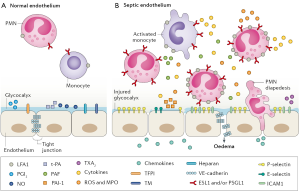
Epithelial cell dysfunction is also a well-known feature of the host response to critical illness. Several abnormalities, including a loss of barrier integrity, increased permeability, epithelial apoptosis and increased levels of biomarkers, have been observed in the lung, liver, kidney and gastrointestinal tract (19). Despite the anatomic closeness of epithelial and endothelial cells, it is unclear to what extent functional disturbances in these two cell types are unique or shared. Many treatments being developed for endothelial dysfunction could also affect similar disturbances in epithelial cells. This might be especially important for understanding how treatments for influenza and Ebola virus disease work, as discussed below.
Statin and ARB effects on endothelial and epithelial dysfunction
Several of the signaling molecules and pathways associated with disrupting or protecting the endothelial barrier are shown in Table 2 (7,12). Treatment with statins and ARBs appears to benefit patients with sepsis, pneumonia, influenza and other forms of critical illness, and may do so by maintaining or restoring endothelial (and perhaps epithelial) barrier integrity. Statins and ARBs are known to affect endothelial cells (for example, 20-24). Their benefits involve (at least in part) the Angpt/Tie2 and ACE2/angiotensin-(1-7)/Mas signaling axes.

Full table
Angpt/Tie2 signaling
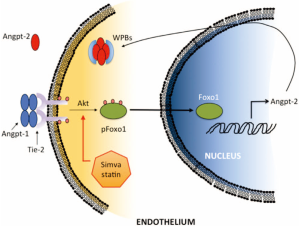
The molecular biology of the Angpt/Tie2 signaling axis has been revealed in studies spanning more than a decade (25-28). Angpt-1 is found in platelets, pericytes and especially in endothelial cells, where it acts constitutively to maintain endothelial barrier integrity. Tie2, a tyrosine kinase receptor, is found almost exclusively in endothelial cells. (There is no information on whether Angpt/Tie2 signaling is involved in any form of epithelial dysfunction in critical illness.) An increase in pro-inflammatory cytokines in patients with sepsis causes the release of Angpt-2 from storage in Weibel-Palade bodies (WPBs) within endothelial cells (Figure 2) (29). Release of Angpt-2 suppresses Tie2 signaling, reduces Foxo-1 phosphorylation and increases Angpt-2 gene transcription. The loss of Tie2 signaling leads directly to endothelial dysfunction, and in mice its restoration reverses the acute lung injury seen with influenza virus infection (30). Increases in RhoA kinase and myosin light chain kinase activity are other mechanisms involved in the loss of endothelial barrier integrity and the ensuing plasma leakage (26-29,31).
In mice (27,28,32) and humans (27-29,31-37), sepsis is associated with increased plasma levels of Angpt-2, increased plasma leakage into the lungs, and increased liver and renal dysfunction (27-33). Tie2 activation maintains or restores endothelial barrier integrity in experimental sepsis (34). In patients with sepsis, an increased serum level of Angpt-2 serves as a useful biomarker that signifies an increased risk of multi-organ failure and death (35-39).
Treatment with statins and ARBs directly affects the Angpt/Tie2 signaling axis. Statins activate Akt and increase phosphorylation of Foxo1, which decreases Angpt-2 production (Figure 2) and increases endothelial barrier integrity (29). Endothelial barrier integrity is also enhanced by statin inhibition of Rho and Rac geranylgeranylation (30). Moreover, mice with a deletion in vitamin D receptor develop severe LPS-induced acute lung injury, which can be reduced by pretreatment with either an experimental Angpt-2 antagonist or an ARB (40).
ACE2/angiotensin-(1-7)/Mas signaling
Studies published more than a decade ago showed that ACE2 is the functional receptor for SARS coronavirus (41-43). Soon thereafter, ACE2 was shown to protect mice from acute lung injury associated with experimental sepsis (44). This study suggested a broad role for ACE2 signaling in the pathogenesis of sepsis, acute lung injury and other forms of acute critical illness (45-47).
The renin-angiotensin system (RAS) modulates many essential functions that maintain homeostasis. Angiotensin II (Ang II), which acts on type 1 Ang II receptor (AT1R), is central to RAS activities. Ang II increases endothelial barrier permeability (48). The ACE homolog ACE2 acts as a counter-regulator to the activities of Ang II by forming angiotensin-(1-7), which acts via the Mas receptor to oppose the vasoconstrictor and inflammatory effects of ACE/Ang II/AT1R signaling (45-47). ACE2 has been found in alveolar epithelial cells, enterocytes in the small intestines, and endothelial and smooth muscle cells in several organs (43).
The ACE2/angiotensin-(1-7)/Mas signaling axis modifies several aspects of both acute and chronic inflammation. It has beneficial effects on insulin metabolism (49) and on the cardiovascular (46,50), renal (51), and coagulation systems (52). It attenuates inflammatory damage to endothelial cells in vascular disease (53-55) and diabetes (56,57). In experimental sepsis, ACE2/ang-(1-7)/Mas signaling down regulates inflammatory changes in endothelial cells (58) and improves outcomes (59,60). Similarly, in experimental acute lung injury, this signaling axis reverses endothelial and epithelial cell dysfunction (61-65) and prevents ARDS (66-68). Children with ARDS have an increase in bronchoalveolar fluid ACE and a decrease in ACE2 (69).
Several experimental studies have demonstrated the benefits of treatments that target ACE2/angiotensin-(1-7)/Mas signaling. In experimental rodent models of sepsis and acute lung injury, ARBs down regulate numerous pro-inflammatory cytokines and improve outcomes (59,62,70). These findings are supported by other studies of ARB treatment in models of cardiac hypertrophy and vascular injury (71-74). Moreover, in a rabbit model of atherosclerosis, statin treatment up regulates ACE2 in the heart, and this is associated with epigenetic histone modifications (75).
Clinical experience with statin and ARB treatment in patients with critical illness
The demonstrated effects of statin and ARB treatment on Angpt/Tie2 and ACE2/angiotensin-(1-7)/Mas signaling in endothelial and epithelial cells provide a solid basis for considering using these drugs to treat patients with acute critical illness. Thus far, statins and ARBs have been used to treat patients with sepsis, ARDS, influenza and Ebola virus disease. Opinions vary on the interpretation of published reports, but on the whole the results have been encouraging.
Sepsis and pneumonia
The experimental foundation for treating the host response in patients with sepsis and acute lung injury can be found in studies of simvastatin treatment of LPS-treated mice (76-78). There have been more than 25 observational studies of outpatient statin treatment in patients hospitalized with infection, pneumonia or sepsis (79). Overall, statins were found to reduce episodes of hospitalization and mortality, although a high degree of heterogeneity among the studies has led to caution in interpreting the results.
Four reports of randomized controlled trials (RCTs) of inpatient statin treatment of ICU patients with sepsis and ARDS have been published (80-85). In three of these reports, patients were mechanically ventilated. None of the RCTs showed a reduction in mortality, and three trials were abandoned early for reasons of futility. Statin treatment was found to be safe in these ICU patients, but it was probably “too little too late”; in one study, patients had been treated with mechanical ventilation for a mean of eight days before statin treatment was begun (81).
Another RCT of statin treatment in sepsis patients was conducted by clinicians in Birmingham, UK (86), although their study has received little attention from intensive care specialists. Patients who presented to the emergency department with signs of sepsis but no evidence (yet) of multi-organ failure were randomized to receive either atorvastatin (49 patients; 40 mg per day) or placebo (51 patients). All study subjects had been statin-naive for at least two weeks, and treatment was started as soon as they were hospitalized. The primary goal was to determine whether “statin treatment might reduce the absolute rate with which sepsis converted to severe sepsis” (i.e., multi-organ failure) (86). The investigators calculated they would need 414 patients to detect a statistically significant reduction in severe sepsis of 15% (e.g., from 40% to 25%). Although slow recruitment forced the investigators to stop their trial prematurely, they succeeded in achieving their primary goal: the occurrence of severe sepsis was reduced not by 15% as planned, but by 83%. Because the development of multi-organ failure in sepsis patients is thought to be due to the loss of endothelial barrier integrity, it is reasonable to conclude that the positive outcome in this RCT reflected the activity of atorvastatin in maintaining endothelial barrier integrity. As discussed above, statins probably contribute to maintaining endothelial barrier integrity by affecting the Angpt/Tie2 signaling axis (30).
Influenza
The host response to influenza virus infection has been studied extensively, and many of these studies have been recently reviewed (87-92). In both experimental and human influenza, a greater degree of virus replication is generally associated with greater inflammation and more severe disease (93,94). However, individual influenza viruses may differ in the extent to which they elicit inflammatory responses, and the degree of hypercytokinemia is not always directly associated with levels of virus replication or mortality (88,95).
Endothelial and epithelial cells are intimately involved in the host response to influenza virus infection, and it is often difficult to separate the two. Both cell types can be infected in vitro with influenza A H5N1 viruses (96-98), and this is associated with a brisk and unbalanced inflammatory response involving (among other things) NF-kappa B and interferon regulatory factor (IRF) 3 (99,100). Studies in cell co-culture systems, however, show that influenza viruses primarily target epithelial rather than endothelial cells (97,101,102). Nonetheless, while helpful, these in vitro studies cannot take account of the complex interplay between these cells and other components of the host response in vivo (especially leukocytes and macrophages), which contribute to the inflammatory response (102-106). Tellingly, the importance of host factors, not virus replication, was shown conclusively in a study of experimental acute lung injury following intra-tracheal instillation of inactivated H5N1 influenza virus (107).
In the lung, ACE2 is found primarily in epithelial cells (108), and the ACE2/angiotensin-(1-7)/Mas axis directly regulates epithelial cell survival (109,110). In mice, ACE2 is a mediator of the acute lung injury caused by influenza A H5N1- and H7N9-virus infection (111,112), and in patients, increased ACE2 levels are associated with severe disease (111,113). In mice experimentally infected with H5N1 influenza, treatment with an ARB (losartan) improves survival (114).
The Angpt/Tie2 signaling axis is also involved in the pathogenesis of experimental influenza (32). Angpt-like 4, a member of the Angpt family that does not signal through Tie2, also promotes vascular permeability in experimental influenza (115). Importantly, a specific Tie2 agonist (Vasculotide) promotes survival in mice infected with influenza virus (31). In this study, all of the mice that were given only an antiviral agent immediately after infection died, whereas those that were also given a Tie2 agonist starting as late as five days after infection survived.
Statins reduce influenza virus replication in vitro (116), but for several reasons, studies of statin treatment of influenza virus-infected mice have been inconclusive (117). There is still a good possibility that statins might contribute to improved survival in human influenza because, among other things, they down regulate pro-inflammatory cytokines. Statins probably act in other ways as well. For example, in mice, TNF-related apoptosis-inducing ligand (TRAIL) signaling promotes apoptosis in epithelial cells (118), while in humans with acute coronary syndrome (another inflammatory illness), statins reduce endothelial cell apoptosis (119). Thus, statins would probably have a positive effect on the host response to influenza. In all of these studies, statins were used by themselves, and not combined with other immunomodulatory agents.
More than a decade ago, when the prospect of a global avian H5N1 influenza pandemic was of great concern, it was suggested that treating the host response might be an effective way to mitigate pandemic mortality (120-122). The need for such an approach was amply demonstrated during the influenza H1N1 pandemic in 2009-2010, when more than 90% of the world’s people had no access to adequate supplies of pandemic vaccines and antiviral agents (121,122). More recently, the emergence of the influenza A H7N9 and similar influenza viruses has served as a reminder that the pandemic threat has not disappeared (123). There are many ongoing efforts to improve the global supply of influenza vaccines (124-126). Nonetheless, during the first 6 months of the next pandemic, access to pandemic vaccines will be severely limited for virtually everyone in the world (121,122).
Influenza accounts for a substantial proportion of cases of medically attended acute respiratory infection (MAARI). Two large observational studies of statin treatment in MAARI patients have yielded conflicting results (there are no such studies for ARBs). In one study from the UK, statin treatment resulted in a 33–35% reduction in 30-day all-cause mortality (127), although the proportion of these illnesses that were caused by influenza virus infection was unknown and the investigators were unable to control for previous influenza vaccination in their analysis. In another study from the US, the effectiveness of influenza vaccination in MAARI patients was actually reduced in individuals who were taking statins (128), although like the UK study, the proportion of illnesses caused by influenza virus infection was unknown. Nonetheless, these findings from the US are compatible with those of another study, which showed that elderly patients taking statins had reduced antibody responses following influenza vaccination (129).
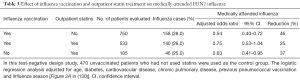
In a recent report, investigators used a test-negative design to study the effectiveness of influenza vaccination, outpatient statin treatment, or both in preventing laboratory-confirmed, medically attended influenza in adults ≥45 years in age (130). Table 3 summarizes their findings for influenza A H3N2. (Similar findings were reported for influenza A H1N1 and influenza B, although the numbers of such patients were small.) In patients who had received influenza vaccine, outpatient treatment with statins reduced the effectiveness of vaccination from 46% to 25%. However, in unvaccinated subjects, outpatient statin treatment alone reduced the number of cases of influenza by 37%. When influenza vaccines are unavailable, as will occur during the early months of the next pandemic, this finding will take on special meaning.
Full table
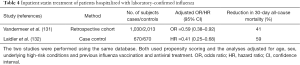
Interpretation of the results of outpatient studies of statin treatment in patients with MAARI and laboratory-confirmed influenza may be debatable, but it is more important to know whether statins benefit patients hospitalized with laboratory-confirmed influenza because it is hospitalized patients who are at increased risk of dying (121). Two observational studies suggest that inpatient statin treatment could reduce 30-day all-cause mortality (Table 4) (131,132). Both studies were conducted using the same database, both used propensity scoring and both controlled for age, sex, underlying co-morbid conditions, and (importantly) previous influenza vaccination and antiviral treatment. The first study (retrospective cohort) found that statin treatment reduced 30-day all-cause mortality by 41%, suggesting that statins might be useful in treating influenza patients requiring hospitalization (Table 4) (131). The second study (case-control) found that statins reduced 30-day mortality by an even greater margin −59% (Table 4) (132). Nonetheless, investigators for the second study concluded that because of unmeasured confounding, statins should not be used “as adjunct treatment for preventing death among persons hospitalized for influenza” (132). The findings for statins and ARB treatment of community-acquired pneumonia (Table 1) and those for statin treatment of laboratory-confirmed influenza (Table 3) suggest this conclusion is premature.
Full table
Ebola virus disease
More than a decade ago, Ebola scientists noted clinical similarities between Ebola and septic shock (133). When the outbreak of Ebola virus disease appeared in West Africa in 2014, experience with treating the host response in patients with sepsis, pneumonia and influenza suggested the same approach might improve outcomes in Ebola patients.
Recent experimental studies suggest that statins might be effective against the Ebola virus itself. For example, heme-oxygenase-1 (HO-1) has been shown to suppress Ebola virus replication (134), and statins are known to up regulate HO-1 (135). Moreover, Ebola viruses are cytotoxic because they target cell membrane cholesterol (136), which is affected by statins. Other studies, however, suggest that the host response to Ebola virus infection itself could be a target of treatment. Genetic studies of Ebola in mice have shown that endothelial dysfunction and increased vascular permeability are associated with Tie2 (137). Ebola virus glycoprotein activates endothelial cells in vitro via a TLR4-mediated mechanism (138), and TLR4 is down regulated by statin treatment. Most of these experimental findings were published before Ebola virus-infected health care workers were evacuated to the US and Europe. Clinical findings documented in the case reports for these patients convincingly demonstrated endothelial dysfunction and massive fluid losses that reflected a breakdown of endothelial barrier integrity (139,140).
On August 15, 2014, shortly after WHO declared Ebola to be a Public Health Emergency of International Concern, a letter was published online suggesting that statins and/or ARBs might be useful in treating Ebola patients and should be tested (141). This idea was not well received by Ebola scientists (142) and officials at the World Health Organization (WHO) (DS Fedson, unpublished observations). Nonetheless, a few months later, physicians in Sierra Leone were able to treat consecutively approximately 100 Ebola patients with a combination of atorvastatin (40 mg/day) and irbesartan (150 mg/day) (143,144). Some of these patients were also treated for 2–3 days with clomiphene, a selective estrogen receptor modifier (SERM) previously shown to have antiviral activity against Ebola virus (145,146). [Although clomiphene has not been described as having immunomodulatory activities, other drugs in the same category have been used to treated immune-mediated diseases (147)]. There was no financial or logistical support for a proper clinical trial, but thanks to a private donation the drugs were made available to local physicians. Only three inadequately treated patients are known to have died (143,144). The physicians and supervising health officials responsible for these patients refused to publicly release information on their experience, but their treatment results were documented immediately in letters and memoranda (Figure 3) and eventually in local newspapers (148). Unfortunately, international organizations involved in the Ebola response, including WHO, have made no effort to validate these findings (DS Fedson, unpublished observations).

Earlier, in September 2014, health officials at WHO decided that only investigational treatments that target the virus would be tested in Ebola patients (149) (experimental Ebola vaccines were also approved for testing). Several clinical trials were undertaken (at great expense) to test experimental antiviral drugs, convalescent plasma and monoclonal antibodies (150). Because of a decline in the number of cases, some of the trials had to be abandoned. In the few that were completed, none of the treatments had a major effect in improving overall survival in Ebola patients, especially in those with high virus loads; i.e., those most in need of effective treatment (151-154).
In spite of these disappointing clinical experiences, Ebola scientists continue to focus on developing treatments that target the virus (155,156). Some investigators insist that the rhesus macaque non-human primate (NHP) model provides the “gold standard” for testing investigational Ebola treatments (157). Surprisingly, no investigator has ever shown that Ebola virus infection in NHPs replicates the endothelial dysfunction and massive fluid losses that are the hallmark of human Ebola virus disease. This contrasts with the experience of earlier investigators who showed that NHPs could be used to study endothelial dysfunction in other models of critical illness (158-160). Moreover, Ebola scientists seem to be unfamiliar with the findings of HIV scientists who have studied simian immunodeficiency virus (SIV) infection in two NHP species. Clinical outcomes are very different in rhesus macaques, all of whom die, compared with sooty mangabeys, all of whom live (161,162). Remarkably, both species have similarly high virus loads. The findings from SIV infection in these two NHP models demonstrate convincingly that the host response, not virus load, primarily determines outcome.
Investigators have yet to explain why gastrointestinal endothelial dysfunction is so prominent in Ebola virus disease, whereas the lung, liver and kidneys are the primary targets of severe or fatal sepsis, pneumonia and influenza. [Interestingly, influenza viruses naturally cause gastrointestinal infection in waterfowl, and experimental GI infection of cats shows that influenza A H5N1 virus targets intestinal endothelial cells and leads to widespread systemic infection (163)].
The complex relationship between the gut and other organs in critical illness has only begun to be explored (164). Experimental and human studies show that ACE2 in gut epithelial cells is necessary for normal absorption of amino acids (165,166) and glucose (167). In addition, the absence of ACE2 in gut epithelial cells alters immunity and leads to increased inflammation (165). Both experimental animals (168) and humans (169) with acute intestinal inflammation have elevated plasma levels of ACE2 and angiotensin-(1-7). In animal models of acute intestinal inflammation, treatment with ARBs down regulates pro-inflammatory cytokines and reduces oxidative stress and epithelial apoptosis (170-172), and statins have similar effects (173-175). Apoptosis of intestinal epithelial cells is a common finding in human and animal studies of critical illness (164). It is tempting to speculate that the response of Ebola patients in Sierra Leone to combination statin/ARB treatment might have been due to the favorable effects of ARBs (and perhaps statins) on ACE2 in intestinal epithelial (and perhaps endothelial) cells.
Clinical investigators continue to seek a better understanding of the host response to Ebola virus infection. A recent study of seven Ebola virus-infected healthcare workers explored 54 separate plasma biomarkers of severe disease (176). The investigators documented activation of the coagulation cascade and evidence of endothelial dysfunction, although levels of Angpt-2, ACE2 and angiotensin-(1-7) were not measured. These biomarker findings reflect the increase in vascular permeability that clinicians have long known to be a central feature of Ebola virus disease.
A few investigators believe that treating the host response in Ebola patients is a reasonable idea (177,178), but their views are not widely shared. Recently, other investigators reviewed the overall clinical experience in managing patients with Ebola virus disease in and outside of West Africa (179). They noted that open-label; uncontrolled studies like the statins/ARB experience in Sierra Leone (143) “precluded any conclusions” about the effectiveness of this treatment. Because no specific treatment had yet been shown to be effective, they concluded, “improving the global capacity to provide supportive critical care… may be associated with the greatest opportunity to improve patient outcomes” (179). They offered no suggestions on how or at what cost this could be accomplished.
Implications for treating the host response to other emerging virus infections
The threat of a major influenza pandemic has not disappeared. Virologists regularly document the natural emergence of influenza virus reassortants with pandemic potential (180). According to WHO, several other emerging viruses also threaten to cause epidemics or pandemics, and urgent attention should be given to developing vaccines and treatments (Table 5) (181). In practical terms, it is unlikely that specific vaccines will be developed for each of these viruses, let alone produced, distributed and administered to populations before one of these viruses emerges. The same is true for specific antiviral agents. Yet the diseases associated with all but one of the emerging viruses requiring urgent research and development (R&D) are characterized by endothelial dysfunction (there are no data for Rift Valley fever) (42,182-185). Endothelial dysfunction is also seen in dengue and Hantavirus pulmonary syndrome (186), and it is a feature of several diseases thought to represent bioterrorist threats, including inhalation anthrax (32,187).
As discussed earlier, a large number of agents that appeared promising in experimental studies have not been found to be effective in clinical trials (2). The recent failure of RCTs of statin treatment to improve outcomes in mechanically ventilated sepsis patients (85) has caused many observers to abandon hope that statins will be effective in treating any form of acute critical illness. In the absence of clinical trial evidence showing that treating the host response actually works, these experiences remind us to remain cautious. The failure of corticosteroid treatment to reduce mortality in patients with severe influenza is well known (188). A recent RCT of statins in adults with dengue was unsuccessful (189). A trial of a TLR4 antagonist failed in patients with severe sepsis (190,191). An exploratory RCT of aspirin treatment of patients at risk of developing ARDS was abandoned because treatment was ineffective (192,193). Several factors could explain why these trial results were disappointing, including a low level of severe disease and mortality in the patient populations being studied (189). In addition, most of these trials were based on earlier experimental or observational studies that examined single agent treatment. Thus, another reason for their failure might be that none of them studied combination treatment with more than one drug.
Treatments that target the infecting virus often bring modest benefits at best, as shown by the clinical trials in Ebola patients discussed above. A study of neuraminidase inhibitors in patients hospitalized during the influenza A H1N1 pandemic reduced overall hospital mortality by 19%, although mortality was reduced by approximately 50% in those who were treated within 2 days of symptom onset, something difficult to achieve in routine clinical care (194). By comparison, numerous experimental studies have shown that modifying the host response (e.g., cytokine knock-out) can dramatically improve survival without having any effect on virus load (121).
Treating the host response with drugs that target endothelial (and perhaps epithelial) dysfunction represents an alternative and potentially more effective strategy for managing of a wide variety of emerging virus diseases (195). There is a good chance that a small number of inexpensive, generically produced and widely available drugs that modify common features of the host response to each of these viruses could be used in the syndromic treatment of them all (121,122,143,144).
Implications for treating the host response to other acute infectious diseases
Observational studies suggest that treating the host response improves outcomes in patients hospitalized with sepsis, pneumonia and influenza, as discussed above. Because the host response to these diseases involves similar or overlapping mechanisms related to endothelial dysfunction, these findings suggest that in addition to treating emerging virus diseases, drugs like statins and ARBs might be used to treat patients with other, acute “everyday” infectious diseases in which similar mechanisms are involved. Some of these diseases occur only occasionally [e.g., Hantavirus pulmonary syndrome (15,186) or inhalation anthrax (187)], but others are far more common, especially in low-income countries. One of them is malaria.
Clinical studies of severe and cerebral malaria (both falciparum and vivax) in children and adults treated with antimalarial drugs have documented endothelial dysfunction: decreased plasma levels of angpt-1, increased levels of angpt-2 and increased angpt-2/angpt-1 ratios (196-203). Other findings include elevated levels of several pro-inflammatory cytokines and chemokines and other biomarkers indicating endothelial [vascular/intercellular adhesion molecule-1 (VCAM-1/ICAM-1), endothelial selectin (E-selectin)), coagulation (thrombomodulin) and complement (C5a) activation.
In experimental studies of cerebral malaria, mice infected with Plasmodium bergei ANKA develop neurological signs five to six days after infection. At the same time, signs of inflammation (increased pro-inflammatory cytokines and chemokines) and endothelial dysregulation become prominent. Under conditions where antimalarial treatment only partially reverses the course of illness, additional treatment with a statin (204-206) or another intervention (207,208) that affects endothelial cells reduces neuro-inflammation, restores endothelial barrier integrity, and increases survival. Dysregulation of the Angpt/Tie2 signaling axis is also seen in experimental P. berghei infection (32,209-211). Treatment with an antimalarial drug combined with either angopoietin-1 (210) or an ARB (irbesartan) (211) restores endothelial barrier integrity and improves survival.
These clinical and experimental finding suggest that adjunctive treatments targeting endothelial dysfunction might improve outcomes in patients with severe and cerebral malaria (198,199). Earlier studies had shown that decreased endothelial nitric oxide (NO) was associated with severe and fatal malaria, but a recent RCT of inhaled NO in malaria patients showed no effect on plasma levels of angpt-2, parasitemia or mortality (212). Given the response of Ebola patients to statin/ARB treatment discussed above, this combination should be considered for inclusion in a clinical trial of adjunctive treatment in malaria patients.
Two additional observations in malaria patients may be relevant for Ebola treatment. First, endothelial dysfunction and systemic inflammation persist for many weeks after antimalarial treatment has cleared the circulation of parasitized red blood cells, and similar findings have been seen following non-malarial infections (213). These observations suggest that persistent endothelial dysfunction might contribute to post-Ebola syndrome and could be a target for treatment.
Second, in a large study of Ebola patients, all of whom received antimalarial treatment with artemether-lumefantrine, the presence of parasitemia was associated with a significant 20% increase in survival (214). This finding was independent of age and Ebola virus load, and the highest level of parasitemia was associated with an 83% survival rate. Several explanations for this “remarkable phenomenon” were suggested, including early up regulation of IFN-β, induction of NK cells, and dampening of the Ebola virus-induced cytokine storm (214). In an earlier study of Ebola patients, all of whom were given antimalarial treatment; those who were treated with artesunate-amodiaquine had a 31% lower risk of mortality compared with those treated with artemether-lumefantrine (215). However, in mice infected with a mouse-adapted Ebola virus, treatment with all four antimalarials, either alone or in combination, had no effect on survival (214), indicating the drugs had no direct antiviral effect. Interestingly, artesunate is known to have broad immunomodulatory activities against microbial infections and inflammatory disorders. In a study of cerebral malaria in P. berghei-infected mice, artesunate (but not mefloquine) improved survival by a mechanism that was independent of its antimalarial activity (216). In studies using murine VE cells, artesunate (I) inhibited NF-kappa B translocation to the nucleus; (II) down regulated the expression of ICAM-1; and (III) prevented parasitized red blood cells from attaching to endothelial cells. Taken together, these observations suggest that in Ebola patients, pre-existing malaria parasitemia might have provided a measure of protection against the effects of Ebola virus on endothelial barrier integrity by activating endothelial defence mechanisms (e.g., up regulating angpt-1 and down regulating angpt-2). Artesunate treatment itself might have had an additional, independent effect on endothelial barrier integrity. These mechanisms are plausible in light of the response of Ebola patients to statin/ARB treatment, a regimen that affects the Angpt/Tie2 signaling axis and improves endothelial barrier integrity.
Ebola and “lessons learned”
In evaluating the response to the H1N1 influenza pandemic in 2009, a WHO report concluded, “The world is ill prepared to respond to a severe influenza pandemic or to any similar global, sustained and threatening public health emergency” (217). The response to the Ebola outbreak in West Africa five years later showed that little had changed (218).
Many commentaries have examined the shortcomings of the international Ebola response, especially the response by WHO (e.g., 219,220). Four independent “lessons learned” reports have offered overlapping recommendations on how to structure a better WHO and international response for managing future epidemics and pandemics (221-224), and published summaries highlight their common features (225,226). Each report recommends strengthening national health systems and reorganizing and improving WHO’s governance and capacity to respond to emergencies. All of them recommend that WHO establish an independent Center for Emergency Preparedness and Response and create a contingency fund to support the rapid deployment of emergency response capabilities (225). They also emphasize the importance of system-wide accountability (e.g., WHO, the United Nations and The World Bank) to ensure an effective global response to future health emergencies.
The four “lessons learned” reports also offer several recommendations for accelerating R&D, and in each report WHO is expected to play a major role (225). WHO has developed its own R&D Blueprint that aims to “reduce the time between the declaration of an international public health emergency and the availability of effective tests, vaccines, antivirals and other treatments…” (227). This R&D Blueprint will serve as “a convening mechanism for public health officials, scientists, and product developers, and an instrument to articulate technical guidance for R&D preparedness and response” (227). In doing so, WHO hopes to fulfil its “global mandate to set evidence-based priorities and standards for research, ensuring that all voices are heard and avoiding conflicts of interest” (227).
The most detailed recommendations for R&D can be found in the report of the Commission on a Global Health Risk Framework for the Future (223). The GHRF Commission calls for establishing a Pandemic Product Development Committee (PPDC) that will be accountable to a Technical Governing Board under the supervision of WHO and it’s Director-General. The PPDC would seek the participation of national regulatory authorities, industry, research organizations, and other public and private stakeholders to promote regulatory convergence, pre-approve clinical trial designs, manage intellectual property and product liability, and expedite the manufacturing and distribution of vaccines, treatments and diagnostics. Adequate funding for this enterprise will be essential, and might require establishing a new global financing mechanism for innovation (228). Two reports suggest this effort would cost at least $1 billion per year (223-225).
None of the Ebola “lessons learned” reports mentions the statins/ARB experience in Sierra Leone and what it might mean for clinical trials of treatments that target the host response (218-230). Instead, the reports call for R&D on new treatments that seem to focus exclusively on new, experimental agents that target only the virus and will undoubtedly require extensive investment (221,223-225). Moreover, the recommendations for coordinating this research call for a prominent role for WHO, which firmly rejected the idea of studying treatments that target the host response to Ebola and which has never convened a meeting to provide “technical guidance for R&D preparedness and response” that includes treating the host response (122,149,227; DS Fedson, unpublished observations). Not surprisingly, WHO has realistically concluded “most individual funding agencies are likely to make decisions on a case by case basis, in line with their mandates and mission” (227).
Several Ebola experts have objected to the publication of reports describing the treatment experience in Sierra Leone (143,144), saying these reports do not constitute evidence of protection (DS Fedson, unpublished observations). These critics have not explained why they have opposed undertaking clinical trials to obtain the data they now demand, or why they have shown no interest in validating what reportedly happened to Ebola patients who were treated in Sierra Leone.
Clinical trials of new treatments
Testing new treatments in the midst of a severe outbreak is fraught with difficulty. The clinical trials that were undertaken during the Ebola outbreak required large-scale international collaboration and were very expensive (151-153,229). One notable development was expansion of the activities of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) (229,230). ISARIC developed a set of pre-approved and adaptable syndrome-based protocols for clinical research and clinical trials that are available online to its member networks in Africa and beyond. ISARIC and its affiliated Platform for European Preparedness Against (Re-)emerging Epidemics (PREPARE) have developed relationships with funders and other organizations that support training and capacity development (230). These developments address many of the administrative, regulatory, legal and ethical difficulties that could otherwise impede a rapid research response during future outbreaks.
When testing new treatments in the setting of an Ebola-like outbreak, a classical 1:1 RCT will seldom be appropriate or even feasible, so choosing an alternative trial design becomes vitally important. As noted recently, “While the scientific merits of RCTs are powerful, advocates for them have not paid enough attention to competing factors that shape research ethics, such as the need for clinical equipoise in starting an RCT, the weakness of knowledge gained when small sample sizes prevail and available supportive care is given to all, the importance of rapidly finding what agent is best among competitors rather than insisting on starting from scratch as RCTs do, and retaining community trust in participating in any RCT with a control arm. Addressing these concerns is crucially important if any trials are to succeed in very challenging circumstances” (231). All of these issues became evident during the recent Ebola clinical trials.
During the Ebola outbreak, a consensus emerged in favor of adaptive trial designs that would minimize the number of patients who would receive less than effective treatment and at the same time accelerate the discovery of one or more treatments that would best improve patient survival (232-234). The statins/ARB treatment experience in Sierra Leone suggests, however, that an adaptive trial design might not be needed. Historical observations show that the effects of many treatments can be so dramatic that bias can be ruled out (235); in these instances, “the observations speak for themselves” (236). The question that must then be asked is “how much difference between treatment outcome and the natural outcome is enough?” (236). Simulation studies show that “implausibly large associations, both between treatment and confounding factor and between confounding factor and outcome, are generally required to explain risks beyond relative rates of 5–10”, and “rate ratios beyond 10 are highly likely to reflect real treatment effects” (236). Compared with historical controls, survival rates in Ebola patients treated with the statin/ARB combination were of this order of magnitude (143,144). Thus, it could be argued that in a future outbreak of Ebola (or Ebola-like disease), an adaptive design clinical trial of a statin/ARB-like treatment regimen might not be needed.
If nothing else, the experience in Sierra Leone suggests that the clinical equipoise needed to justify a RCT of a treatment targeting the host response to Ebola virus infection may no longer exist. Moreover, RCTs “bring final quantification, (but) they offer little scientific novelty in themselves. Before an idea can be confirmed or quantified, it has first to be discovered. For true intellectual advancement, i.e., in proposing new problems, new solutions, or new ideas… (it is case reports and case series that suggest)… mechanisms (and) therapeutic surprises” (237). The case series of Ebola patients who were treated with a statin/ARB combination constitutes such a surprise.
Suggestions for treatment research
There is an obvious need for effective drugs to treat each of the emerging virus diseases that threaten to cause epidemics or pandemics. Important lessons have been learned from studies of treatments for influenza, sepsis, pneumonia and Ebola, as discussed above. Most of these studies have been observational in design, which means that compared with RCTs they have well recognized limitations (e.g., healthy user bias, confounding by indication and other unmeasured confounding variables) (238-240). Nonetheless, physicians know that “nearly all clinical decisions involve probabilistic reasoning, …so a realistic goal for observational research may not be the high standard set by RCTs but instead the level of certainty needed to influence a … treatment decision” (240). This admonition is especially important given the short time frame (a few weeks at most) for studies that evaluate treatment outcomes in acute critical illness.
Ebola follow up and post-Ebola syndrome
A recurrence of acute Ebola virus disease is still possible, and plans should be made for clinical studies of experimental and generic treatments if this occurs. In the meantime, the statin/ARB treatment experience in Sierra Leone should be evaluated and the findings independently validated (143,144). If validated, a conventional clinical trial of statin/ARB treatment might not be necessary (see discussion above).
The dimensions of post-Ebola syndrome also need to be defined: the duration of virus persistence and the long-term effects of uveitis, arthritis, and neurologic complications (241). Several follow up studies to do this are now underway. Although it is unclear whether antiviral agents will be needed to clear the virus, investigators should not overlook the possibility that statins, ARBs and drugs like clomiphene could be useful in treating the post-Ebola complications seen in these patients (242-245). Investigators should also consider the possibility that post-Ebola syndrome might share features in common with chronic critical illness due to other causes (246).
Cellular mechanisms that support virus replication
In clinical studies, treatments that target the virus have been only modestly effective, yet antiviral treatments are still the focus of new research strategies, including drug repurposing (247). If new antivirals must be specific for each individual virus, this approach to research has many disadvantages. One way to overcome this problem might be to discover drugs that target common cellular mechanisms that support virus replication. As proposed for influenza viruses (248), this approach would minimize the possibility of virus resistance, would (presumably) work only in virus-infected cells (see below), would likely reduce unwanted side effects, and could include several drugs that are already licensed for treating other diseases. Suggested cellular targets for influenza antiviral intervention include (I) the Raf/MEK/ERK kinase and p38 MAPK kinase pathways and (II) the IKK/NF-kappaB pathway. Cell culture studies of several unlicensed agents that down regulate these pathways have given promising results (248). Interestingly, there is considerable molecular cross-talk between these two pathways in endothelial cells (249), epithelial cells (250), and macrophages (251). In each of these cells and in rat kidneys in vivo (252), statins down regulate both of these pathways. Statins also prevent Ang II-induced endothelial dysfunction by inhibiting p38 MAPK (253,254). In addition, ARBs inhibit the RAF/MEK/ERK and p38 MAPK pathways (255-257). Finally, in septic mice, giving an ARB 30 minutes after cecal ligation and puncture inhibited NF-kappa B, p38 MAPK and ERK1/2, reduced levels of pro-inflammatory cytokines in lung tissue, and significantly improved survival (258). Considered together, these findings suggest that in influenza and perhaps other emerging virus diseases, treatment with statins and ARBs would have much broader effects on the host response than simply interfering with the cellular machinery necessary for virus replication. These effects deserve to be studied in animal models of these diseases.
Biomarker studies
An important discussion is taking place among critical care investigators regarding the utility of biomarkers in guiding their studies. This discussion has been prompted by (I) the failure of clinical trials in sepsis and ARDS to identify new treatments that will improve patient outcomes (2); and (II) the availability of several new laboratory and computational technologies that allow investigators to identify an increasingly large number of biomarkers that might inform their clinical studies.
Investigators recognize there is substantial variation in the host response to critical illness. To address this heterogeneity, one group of investigators believes that by using the techniques of integrated genomics (e.g., mapping gene expression as expression quantitative trait loci) they can define “subgroups of patients with different immune response states and prognosis … (that will provide) …new insights into the pathogenesis of sepsis and create opportunities for … precision medicine … to target therapeutic inventions and improve sepsis outcomes” (259). These techniques can be used to define patient subgroups that show greater immunosuppression and higher mortality. They are also said to be more precise than definitions based on clinical criteria alone.
Another group of investigators has taken up the challenge of “rethinking strategies” for clinical trial design by contrasting “lumpers” and “splitters” (260). Lumpers assemble heterogeneous groups of patients using simple syndromic definitions, but they are unable to identify subgroups within trial populations that have specific dysregulation of immune pathways or poorer outcomes. By contrast, splitters are interested in identifying specific subtypes in order to “match the right treatment to the right patients” (260). To address these conflicting views, the investigators propose three strategies to improve clinical trial design: practical enrichment (i.e., decrease “noise” in clinical trials), prognostic enrichment (i.e., identify subgroups at higher risk) and predictive enrichment (i.e., identify patients who will respond best to treatment). They believe that “different treatments require different target populations” (260). Because these populations cannot be identified beforehand, they argue that high-throughput searches can be used to identify biomarkers of therapeutic responsiveness that could inform clinical trial design. They believe that “tightly defined criteria enriching for treatment benefit will be the key to advancing treatment for sepsis and ARDS”, and advocate moving beyond a “one-size-fits-all” approach to treatment research (260).
Although the discussion of the role of biomarkers summarized above is thoughtful, it is important to ask whether treatment advances that require “greater personalization of care” (260) will be relevant to discovering new ways to treat patients with emerging virus diseases. To be useful, tests for biomarkers must be rapid, highly accurate and inexpensive. Furthermore, investigators must not overlook “evidence-based treatments that are broadly applicable”, and recognize that “substantial investments into disease subtyping have not always translated in effective targeted treatments” (260). In all likelihood, many if not most emerging virus diseases will first (or eventually) affect people in low- and middle-income countries. It is not clear whether an approach to treatment discovery that is based on “precision” or “personalized” medicine will in any practical sense meet their needs.
A different approach to treatment research
The search for ways to treat patients with Ebola virus disease is still dominated by a focus on drugs that target the virus (261,262). The Ebola “lessons learned” reports call for huge investments that (it is hoped) will lead to the discovery of novel therapeutic agents (223-225). The reports call for this research to be coordinated at an international level, with active leadership by WHO. Given the failures outlined in the “lessons learned” reports and the disappointing results from clinical trials of investigational treatments in West Africa (150-154), it is important to ask what should be done when “underperforming big ideas in research become entrenched” (263).
The ideas of “precision” or “personalized” medicine and the technologies on which they are based have become central to the research of many scientists, and they are widely accepted by institutions that support their work. Yet, for all of their complexity and promise, systems biology and personalized medicine have significant intellectual limitations (264-266). Enormous waste has been documented in biomedical (267) and clinical (268) research. Moreover, introducing a new scientific idea can be difficult (269): just ask an investigator who has prepared a grant proposal or tried to publish a paper describing it. Empirical research shows that evaluators of research proposals systematically downgrade those that seek to explore new ideas (270). Social influences, behavioral bias and herding instincts among scientists “distort the evolution of knowledge if scientists are reluctant to accept an alternative explanation for their observations” (269). Instead, a propensity for “cognitive cronyism” (270) tends to exclude new ideas from consideration. Among other things, the reputational and/or financial costs of doing so can be very large.
The laboratory and clinical studies reviewed in this article suggest that an approach to treatment discovery based on the phenotypic benefits observed in patients who have been treated with inexpensive generic drugs like statins and ARBs has much to offer populations that will be affected by emerging virus diseases. This idea has not required RCTs to establish its validity (271). Instead, it is based on an acceptance of the (I) findings of reductionist scientists whose laboratory studies suggest plausible mechanisms for the pathophysiology of these diseases; (II) laboratory evidence of how these treatments work; and (III) observations of physicians and clinical epidemiologists showing that these treatments work in patients with several types of critical illness.
The relevance of this approach for treating patients who in the future might become infected with an emerging virus is based on existing knowledge of pathophysiological mechanisms shared by all of these viruses and how these mechanisms are affected by treatment (272). More specifically, the studies reviewed here focus on (I) the cellular targets of several forms of critical disease (endothelial and epithelial cells); (II) the shared mechanisms of molecular dysfunction found in these diseases (the Angpt/Tie2 and ACE2/angiotensin-(1-7)/Mas signaling axes); and (III) two inexpensive and widely available generic drugs (statins and ARBs) that target the dysfunctional signaling pathways common to these and other diseases caused by emerging viruses. Although endothelial dysfunction has been a principal focus of much of this research (273,274), the diseases themselves are systemic in nature, and the effects of statin and ARB treatment on the host response are widespread and complex.
One area of research that is relevant to all of these diseases and that has received increasing attention is immunometabolism: the study of the relationship between systemic and cellular metabolism and how it affects immune cell function (275,276). Statins and ARBs have broad and well-known anti-inflammatory and immunomodulatory activities (5,6), but they also affect immunometabolic pathways. For example, oxidative phosphorylation and fatty acid oxidation are characteristic of M2 macrophages (276). Statins and ARBs reduce oxidative stress (277), ARBs promote fatty acid oxidation (278), and both are associated with M2 macrophage phenotypes (279,280). Statins affect T cell polarization by favoring T regulatory cells over Th17 cells (276,281). In addition, AMP-activated protein kinase (AMPK) acts as a master regulator of cellular energy balance by maintaining glucose and lipid homeostasis (275). AMPK is up regulated by statins (282) and ARBs (278). These are just a few of the many ways that statins and ARBs affect immunometabolic pathways.
Conclusions
The Ebola experience in West Africa is a reminder of the ongoing threat of emerging viruses that could cause severe epidemics or pandemics. The international response to the Ebola crisis has been widely criticized, not least for the earlier failure to develop vaccines and treatments that could have mitigated its devastating impact. The reasons for this failure are understandable: an outbreak of this magnitude was historically unprecedented, Ebola vaccines and treatments were perceived as having no commercial value, and other ongoing problems (e.g., malaria, HIV/AIDS) demanded attention.
The international response to the Ebola crisis, like the response to the 2009 influenza pandemic, has been to call for a series of “top down” efforts to dramatically upgrade healthcare systems (especially surveillance), and make large-scale investments in internationally coordinated programs to develop new vaccines and treatments. Little thought seems to have been given to the difficulty of choosing which of the many virus threats should receive priority or, if vaccines and treatments could be developed, how they might be used. For example, the much-heralded success of the Ebola vaccine trial in Guinea (283) depended on an existing human infrastructure that made ring vaccination possible: accurate contact tracing and tracing the contacts of all contacts. This human infrastructure will not exist in most countries when a new epidemic or pandemic virus first appears. Moreover, it will be impossible to vaccinate entire populations beforehand when the identity of the virus that might eventually emerge is not known. In all likelihood, these vaccines will first be given to laboratory and healthcare workers, and only later to populations at risk.
The development of new drugs that target emerging viruses also faces significant limitations. If they are to be specific for each virus, the identity of the next epidemic or pandemic virus will not be known, so choosing which drug to develop will be a matter of guesswork. If newly developed antiviral drugs have broad-spectrum activity, they will still be expensive, in limited supply, and unfamiliar to most physicians. Furthermore, they might be no more effective than the agents that were tested against Ebola (150-154).
A conference held in November 2015 considered (once again) the lessons that should be learned from the Ebola crisis and how they might guide the response to future outbreaks (284). In considering the need for coordinated research, experts agreed that an effective response required more than the input from biomedical scientists; it also required the contributions of engineers, social scientists and ethicists. Yet, the examples given for needed research were biomedical: (I) studies of the human-animal interface; (II) contributions of molecular virology and immunology to understanding population herd immunity (this was considered a crucial area for research); and (III) better vaccines and treatments (although only vaccines were discussed). Once again, the emphasis was on “top down” international cooperation and synergistic action led by WHO. There was no mention of the need for research on treating the host response.
The idea of treating the host response to an emerging virus disease is at least a decade old (120-122,285,286), but it has received little or no attention from WHO, other international institutions, and the scientific community (142; DS Fedson, unpublished observations). Nonetheless, phenotypic observations by physicians and clinical epidemiologists suggest that one or more inexpensive and widely available generic drugs could be used to treat patients with many infections that are severe enough to require hospital care (Table 6). These orally administered drugs are known to be safe when given to patients with acute critical illness. Because they are used routinely to treat patients with cardiovascular diseases, they are familiar to physicians everywhere. In addition to treating patients with acute critical illness, experience with outpatient treatment in patients with influenza (Table 3) (130), sepsis (79) and pneumonia (Table 1) (3) suggests that these drugs might also be used in prophylaxis; for example, they could be given to healthcare workers and contacts of patients with Ebola virus disease.

Full table
The laboratory and clinical research needed to study this approach to treatment does not require “top down” international coordination. Many reports show it could be undertaken by individual scientists or groups of investigators, many of whom could and should come from low- and middle-income countries (287). If their research conclusively demonstrates the effectiveness of these drugs, they could be used to treat patients infected with many emerging viruses. Because these drugs are generic and inexpensive, global supplies are huge. Consequently, they could be used in any country that has a basic healthcare system, and treatment could be given on the very first epidemic or pandemic day.
There is a strong possibility that a “bottom up” approach to treatment using inexpensive generic drugs could reduce mortality in the next Ebola-like epidemic or the next influenza pandemic. In doing so, it could make an immeasurable contribution to global health, global equity and global security.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- van Vught LA, Scicluna BP, Wiewel MA, et al. Comparative analysis of the host response to community-acquired and hospital-acquired pneumonia in critically ill patients. Am J Respir Crit Care Med 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med 2014;20:195-203. [Crossref] [PubMed]
- Mortensen EM, Nakashima B, Cornell J, et al. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis 2012;55:1466-73. [Crossref] [PubMed]
- Mortensen EM, Pugh MJV, Anzueto A. Prior use of a statin and ARB is associated with lower mortality for patients hospitalized with pneumonia. Eur Respir J 2016;48 Suppl 60:abstr 3329.
- Tousoulis D, Psarros C, Demosthenous M, et al. Innate and adaptive inflammation as a therapeutic target in vascular disease: the emerging role of statins. J Am Coll Cardiol 2014;63:2491-502. [Crossref] [PubMed]
- Di Raimondo D, Tuttolomondo A, Butta D, et al. Effects of ACE inhibitors and angiotensin receptor blockers on inflammation. Curr Pharm Des 2012;18:4385-413. [Crossref] [PubMed]
- Hotchkiss RS, Moldawer LL, Opal SM, et al. Sepsis and septic shock. Nat Rev Dis Prim 2016;2:16046. [Crossref] [PubMed]
- Quinton LJ, Mizgerd JP. Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling. Annu Rev Physiol 2015;77:407-30. [Crossref] [PubMed]
- Nickenig G. Should angiotensin II receptor blockers and statins be combined? Circulation 2004;110:1013-20. [Crossref] [PubMed]
- Lee HY, Sakuma I, Ihm SH, et al. Statins and renin-angiotensin system inhibitor combination treatment to prevent cardiovascular disease. Circ J 2014;78:281-7. [Crossref] [PubMed]
- Goldenberg NM, Steinberg BE, Slutsky AS, et al. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med 2011;3:88ps25. [Crossref] [PubMed]
- Opal SM, van der Poll T. Endothelial barrier dysfunction in septic shock. J Intern Med 2015;277:277-93. [Crossref] [PubMed]
- Steinberg BE, Goldenberg NM, Lee WL. Do viral infections mimic bacterial sepsis? The role of microvascular permeability: a review of mechanisms and methods. Antiviral Res 2012;93:2-15. [Crossref] [PubMed]
- Teijaro JR, Walsh KB, Cahalan S, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 2011;146:980-91. [Crossref] [PubMed]
- Spiropoulou CF, Srikiatkhachorn A. The role of endothelial activation in dengue hemorrhagic fever and hantavirus pulmonary syndrome. Virulence 2013;4:525-36. [Crossref] [PubMed]
- Huet O, Dupic L, Harrois A, et al. Oxidative stress and endothelial dysfunction during sepsis. Front Biosci (Landmark Ed) 2011;16:1986-95. [Crossref] [PubMed]
- Rocha M, Herance R, Rovira S, et al. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infect Disord Drug Targets 2012;12:161-78. [Crossref] [PubMed]
- Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res 2013;112:1171-88. [Crossref] [PubMed]
- Chawla LS, Fink M, Goldstein SL, et al. The epithelium as a target in sepsis. Shock 2016;45:249-58. [Crossref] [PubMed]
- Jougasaki M, Ichiki T, Takenoshita Y, et al. Statins suppress interleukin-6-induced monocyte chemo-attractant protein-1 by inhibiting Janus kinase/signal transducers and activators of transcription pathways in human vascular endothelial cells. Br J Pharmacol 2010;159:1294-303. [Crossref] [PubMed]
- Hol J, Otterdal K, Breland UM, et al. Statins affect the presentation of endothelial chemokines by targeting to multivesicular bodies. PLoS One 2012;7:e40673. [Crossref] [PubMed]
- Su JB. Vascular endothelial dysfunction and pharmacological treatment. World J Cardiol 2015;7:719-41. [Crossref] [PubMed]
- Ceriello A, Assaloni R, Da Ros R, et al. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation 2005;111:2518-24. [Crossref] [PubMed]
- Willemsen JM, Westerink JW, Dallinga-Thie GM, et al. Angiotensin II type 1 receptor blockade improves hyperglycemia-induced endothelial dysfunction and reduces proinflammatory cytokine release from leukocytes. J Cardiovasc Pharmacol 2007;49:6-12. [Crossref] [PubMed]
- Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000;6:460-3. [Crossref] [PubMed]
- Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 2006;3:e46. [Crossref] [PubMed]
- Milam KE, Parikh SM. The angiopoietin-Tie2 signaling axis in the vascular leakage of systemic inflammation. Tissue Barriers 2015;3:e957508. [Crossref] [PubMed]
- Bomsztyk K, Mar D, An D, et al. Experimental acute lung injury induces multi-organ epigenetic modifications in key angiogenic genes implicated in sepsis-associated endothelial dysfunction. Crit Care 2015;19:225. [Crossref] [PubMed]
- Ghosh CC, Thamm K, Berghelli AV, et al. Drug repurposing screen identifies Foxo1-dependent angiopoietin-2 regulation in sepsis. Crit Care Med 2015;43:e230-40. [Crossref] [PubMed]
- Sugiyama MG, Armstrong SM, Wang C, et al. The Tie-2 agonist Vasculotide rescues mice from influenza virus infection. Sci Rep 2015;5:11030. [Crossref] [PubMed]
- Xiao H, Xiong Q, Ping D, et al. Inhibition of Rho and Rac geranylgeranylation by atorvastatin is critical for preservation of endothelial junction integrity. PLoS One 2013;8:e59233. [Crossref] [PubMed]
- Ziegler T, Horstkotte J, Schwab C, et al. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest 2013;123:3436-45. [Crossref] [PubMed]
- Ghosh CC, David S, Zhang R, et al. Gene control of tyrosine kinase TIE2 and vascular manifestations of infections. Proc Natl Acad Sci USA 2016;113:2472-7. [Crossref] [PubMed]
- Han S, Lee SJ, Kim KE, et al. Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci Transl Med 2016;8:335ra55. [Crossref] [PubMed]
- Calfee CS, Gallagher D, Abbott J, et al. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med 2012;40:1731-7. [Crossref] [PubMed]
- Agrawal A, Matthay MA, Kangelaris KN, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med 2013;187:736-42. [Crossref] [PubMed]
- Mikacenic C, Hahn WO, Price BL, et al. Biomarkers of endothelial activation are associated with poor o utcome in critical illness. PLoS One 2015;10:e0141251. [Crossref] [PubMed]
- Zinter MS, Spicer A, Orwoll BO, et al. Plasma angiopoietin-2 outperforms other markers of endothelial injury in prognosticating pediatric ARDS mortality. Am J Physiol Lung Cell Mol Physiol 2016;310:L224-31. [Crossref] [PubMed]
- Fisher J, Douglas JJ, Linder A, et al. Elevated plasma angiopoietin-2 levels are associated with fluid overload, organ dysfunction, and mortality in human septic shock. Crit Care Med 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Kong J, Zhu X, Shi Y, et al. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol 2013;27:2116-25. [Crossref] [PubMed]
- Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875-9. [Crossref] [PubMed]
- Kuhn JH, Li W, Choe H, et al. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci 2004;61:2738-43. [Crossref] [PubMed]
- Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631-7. [Crossref] [PubMed]
- Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112-6. [Crossref] [PubMed]
- Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol 2013;169:477-92. [Crossref] [PubMed]
- Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J 2013;77:301-8. [Crossref] [PubMed]
- Jiang F, Yang J, Zhang Y, et al. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat Rev Cardiol 2014;11:413-26. [Crossref] [PubMed]
- Bodor C, Nagy JP, Vegh B, et al. Angiotensin II increases the permeability and PV-1 expression of endothelial cells. Am J Physiol Cell Physiol 2012;302:C267-76. [Crossref] [PubMed]
- Dominici FP, Burghi V, Muñoz MC, et al. Modulation of the action of insulin by angiotensin-(1-7). Clin Sci (Lond) 2014;126:613-30. [Crossref] [PubMed]
- Rabelo LA, Alenina N, Bader M. ACE2-angiotensin-(1-7)-Mas axis and oxidative stress in cardiovascular disease. Hypertens Res 2011;34:154-60. [Crossref] [PubMed]
- Kim SM, Kim YG, Jeong KH, et al. Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PLoS One 2012;7:e39739. [Crossref] [PubMed]
- Fraga-Silva RA, Da Silva DG, Montecucco F, et al. The angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas receptor axis: a potential target for treating thrombotic diseases. Thromb Haemost 2012;108:1089-96. [Crossref] [PubMed]
- Lovren F, Pan Y, Quan A, et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol 2008;295:H1377-84. [Crossref] [PubMed]
- Zhang C, Zhao YX, Zhang YH, et al. Angiotensin-converting enzyme 2 attenuates atherosclerotic lesions by targeting vascular cells. Proc Natl Acad Sci U S A 2010;107:15886-91. [Crossref] [PubMed]
- Zhang YH, Zhang YH, Dong XF, et al. ACE2 and Ang-(1-7) protect endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response. Inflamm Res 2015;64:253-60. [Crossref] [PubMed]
- Yuan L, Lu CL, Wang Y, et al. Ang (1-7) protects islet endothelial cells from palmitate-induced apoptosis by AKT, eNOS, p38 MAPK, and JNK pathways. J Diabetes Res 2014;2014:391476.
- Zhang Y, Liu J, Luo JY, et al. Upregulation of angiotensin (1-7)-mediated signaling preserves endothelial function through reducing oxidative stress in diabetes. Antioxid Redox Signal 2015;23:880-92. [Crossref] [PubMed]
- Lund DD, Brooks RM, Faraci FM, et al. Role of angiotensin II in endothelial dysfunction induced by lipopolysaccharide in mice. Am J Physiol Heart Circ Physiol 2007;293:H3726-31. [Crossref] [PubMed]
- Hagiwara S, Iwasaka H, Hidaka S, et al. Antagonist of the type-1 ANG II receptor prevents against LPS-induced septic shock in rats. Intensive Care Med 2009;35:1471-8. [Crossref] [PubMed]
- Schmidt C, Höcherl K, Kurt B, et al. Blockade of multiple but not single cytokines abrogates downregulation of angiotensin II type-I receptors and anticipates septic shock. Cytokine 2010;49:30-8. [Crossref] [PubMed]
- Orfanos SE, Armaganidis A, Glynos C, et al. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in acute lung injury. Circulation 2000;102:2011-8. [Crossref] [PubMed]
- Wong MH, Chapin OC, Johnson MD. LPS-stimulated cytokine production in type I cells is modulated by the renin-angiotensin system. Am J Respir Cell Mol Biol 2012;46:641-50. [Crossref] [PubMed]
- Bao H, Gao F, Xie G, et al. Angiotensin-converting enzyme 2 inhibits apoptosis of pulmonary endothelial cells during acute lung injury through suppressing MiR-4262. Cell Physiol Biochem 2015;37:759-67. [Crossref] [PubMed]
- Li Y, Cao Y, Zeng Z, et al. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis prevents lipopolysaccharide-induced apoptosis of pulmonary microvascular endothelial cells by inhibiting JNK/NF-κB pathways. Sci Rep 2015;5:8209. [Crossref] [PubMed]
- Gopallawa I, Uhal BD. Molecular and cellular mechanisms of the inhibitory effects of ACE-2/ANG1-7/Mas axis on lung injury. Curr Top Pharmacol 2014;18:71-80. [PubMed]
- Wösten-van Asperen RM, Lutter R, Specht PA, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol 2011;225:618-27. [Crossref] [PubMed]
- Li Y, Zeng Z, Cao Y, et al. Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-κB signaling pathways. Sci Rep 2016;6:27911. [Crossref] [PubMed]
- Yu X, Lin Q, Qin X, et al. ACE2 antagonizes VEGFa to reduce vascular permeability during acute lung injury. Cell Physiol Biochem 2016;38:1055-62. [Crossref] [PubMed]
- Wösten-van Asperen RM, Bos AP, Bem RA, et al. Imbalance between pulmonary angiotensin-converting enzyme and angiotensin-converting enzyme 2 activity in acute respiratory distress syndrome. Pediatr Crit Care Med 2013;14:e438-41. [Crossref] [PubMed]
- Dasu MR, Riosvelasco AC, Jialal I. Candesartan inhibits Toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis 2009;202:76-83. [Crossref] [PubMed]
- Iwai M, Nakaoka H, Senba I, et al. Possible involvement of angiotensin-converting enzyme 2 and Mas activation in inhibitory effects of angiotensin II Type 1 receptor blockade on vascular remodeling. Hypertension 2012;60:137-44. [Crossref] [PubMed]
- Zhang ZZ, Shang QH, Jin HY, et al. Cardiac protective effects of irbesartan via the PPAR-gamma signaling pathway in angiotensin-converting enzyme 2-deficient mice. J Transl Med 2013;11:229. [Crossref] [PubMed]
- Ohshima K, Mogi M, Nakaoka H, et al. Possible role of angiotensin-converting enzyme 2 and activation of angiotensin II type 2 receptor by angiotensin-(1-7) in improvement of vascular remodeling by angiotensin II type 1 receptor blockade. Hypertension 2014;63:e53-9. [Crossref] [PubMed]
- Clancy P, Koblar SA, Golledge J. Angiotensin receptor 1 blockade reduces secretion of inflammation associated cytokines from cultured human carotid atheroma and vascular cells in association with reduced extracellular signal regulated kinase expression and activation. Atherosclerosis 2014;236:108-15. [Crossref] [PubMed]
- Tikoo K, Patel G, Kumar S, et al. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol 2015;93:343-51. [Crossref] [PubMed]
- Merx MW, Liehn EA, Graf J, et al. Statin treatment after onset of sepsis in a murine model improves survival. Circulation 2005;112:117-24. [Crossref] [PubMed]
- Jacobson JR. Statins in endothelial signaling and activation. Antioxid Redox Signal 2009;11:811-21. [Crossref] [PubMed]
- Singla S, Jacobson JR. Statins as a novel therapeutic strategy in acute lung injury. Pulm Circ 2012;2:397-406. [Crossref] [PubMed]
- Wan YD, Sun TW, Kan QC, et al. Effect of statin therapy on mortality from infection and sepsis: a meta-analysis of randomized and observational studies. Crit Care 2014;18:R71. [Crossref] [PubMed]
- Novack V, Eisinger M, Frenkel A, et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med 2009;35:1255-60. [Crossref] [PubMed]
- Papazian L, Roch A, Charles PE, et al. STATIN-VAP Study Group. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: a randomized clinical trial. JAMA 2013;310:1692-700. [Crossref] [PubMed]
- Truwit JD, Bernard GR, Steingrub J, et al. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 2014;370:2191-200. [Crossref] [PubMed]
- McAuley DF, Laffey JG, O’Kane CM, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med 2014;371:1695-703. [Crossref] [PubMed]
- Deshpande A, Pasupuleti V, Rothberg MB. Statin therapy and mortality from sepsis: a meta-analysis of randomized trials. Am J Med 2015;128:410-7.e1. [Crossref] [PubMed]
- Thomas G, Hraiech S, Loundou A, et al. Statin therapy in critically-ill patients with severe sepsis: a review and meta-analysis of randomized clinical trials. Minerva Anestesiol 2015;81:921-30. [PubMed]
- Patel JM, Snaith C, Thickett DR, et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial). Crit Care 2012;16:R231. [Crossref] [PubMed]
- Short KR, Kroeze EJ, Fouchier RA, et al. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis 2014;14:57-69. [Crossref] [PubMed]
- Herold S, Becker C, Ridge KM, et al. Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur Respir J 2015;45:1463-78. [Crossref] [PubMed]
- Kash JC, Taubenberger JK. The role of viral, host, and secondary bacterial factors in influenza pathogenesis. Am J Pathol 2015;185:1528-36. [Crossref] [PubMed]
- Gregory DJ, Kobzik L. Influenza lung injury: mechanisms and therapeutic opportunities. Am J Physiol Lung Cell Mol Physiol 2015;309:L1041-6. [PubMed]
- Ramos I, Fernandez-Sesma A. Modulating the innate immune response to influenza A virus: potential therapeutic use of anti-inflammatory drugs. Front Immunol 2015;6:361. [Crossref] [PubMed]
- Peteranderl C, Herold S, Schmoldt C. Human influenza virus infections. Semin Respir Crit Care Med 2016;37:487-500. [Crossref] [PubMed]
- Hatta Y, Hershberger K, Shinya K, et al. Viral replication rate regulates clinical outcome and CD8 T cell responses during highly pathogenic H5N1 influenza virus infection in mice. PLoS Pathog 2010;6:e1001139. [Crossref] [PubMed]
- Lee N, Wong CK, Chan PKS, et al. Cytokine response patterns in severe pandemic 2009 H1N1 and seasonal influenza among hospitalized adults. PLoS One 2011;6:e26050. [Crossref] [PubMed]
- Meliopoulos VA, Karlsson EA, Kercher L, et al. Human H7N9 and H5N1 influenza viruses differ in induction of cytokines and tissue tropism. J Virol 2014;88:12982-91. [Crossref] [PubMed]
- Chan RW, Chan MC, Nicholls JM, et al. Use of ex vivo and in vitro cultures of the human respiratory tract to study the tropism and host responses of highly pathogenic avian influenza A (H5N1) and other influenza viruses. Virus Res 2013;178:133-45. [Crossref] [PubMed]
- Short KR, Veldhuis Kroeze EJ, Reperant LA, et al. Influenza virus and endothelial cells: a species specific relationship. Front Microbiol 2014;5:653. [Crossref] [PubMed]
- Armstrong SM, Mubareka S, Lee WL. The lung microvascular endothelium as a therapeutic target in severe influenza. Antiviral Res 2013;99:113-8. [Crossref] [PubMed]
- Viemann D, Schmolke M, Lueken A, et al. H5N1 virus activates signaling pathways in human endothelial cells resulting in a specific imbalanced inflammatory response. J Immunol 2011;186:164-73. [Crossref] [PubMed]
- Zeng H, Pappas C, Belser JA, et al. Human pulmonary microvascular endothelial cells support productive replication of highly pathogenic avian influenza viruses: possible involvement in the pathogenesis of human H5N1 virus infection. J Virol 2012;86:667-78. [Crossref] [PubMed]
- Chan MC, Chan RW, Yu WC, et al. Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells. Respir Res 2009;10:102. [Crossref] [PubMed]
- Short KR, Kasper J, van der Aa S, et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur Respir J 2016;47:954-66. [Crossref] [PubMed]
- Högner K, Wolff T, Pleschka S, et al. Macrophage-expressed IFN-β contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLoS Pathog 2013;9:e1003188. [Crossref] [PubMed]
- Ichikawa A, Kuba K, Morita M, et al. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am J Respir Crit Care Med 2013;187:65-77. [Crossref] [PubMed]
- Teijaro JR, Walsh KB, Rice S, et al. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc Natl Acad Sci U S A 2014;111:3799-804. [Crossref] [PubMed]
- Peteranderl C, Morales-Nebreda L, Selvakumar B, et al. Macrophage-epithelial paracrine crosstalk inhibits lung edema clearance during influenza infection. J Clin Invest 2016;126:1566-80. [Crossref] [PubMed]
- Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008;133:235-49. [Crossref] [PubMed]
- Wiener RS, Cao YX, Hinds A, et al. Angiotensin converting enzyme 2 is primarily epithelial and is developmentally regulated in the mouse lung. J Cell Biochem 2007;101:1278-91. [Crossref] [PubMed]
- Uhal BD, Li X, Xue A, et al. Regulation of alveolar epithelial cell survival by the ACE-2/angiotensin 1-7/Mas axis. Am J Physiol Lung Cell Mol Physiol 2011;301:L269-74. [Crossref] [PubMed]
- Gopallawa I, Uhal BD. Angiotensin-(1-7)/mas inhibits apoptosis in alveolar epithelial cells through upregulation of MAP kinase phosphatase-2. Am J Physiol Lung Cell Mol Physiol 2016;310:L240-8. [Crossref] [PubMed]
- Zou Z, Yan Y, Shu Y, et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun 2014;5:3594. [Crossref] [PubMed]
- Yang P, Gu H, Zhao Z, et al. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep 2014;4:7027. [Crossref] [PubMed]
- Huang F, Guo J, Zou Z, et al. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat Commun 2014;5:3595. [Crossref] [PubMed]
- Yan Y, Liu Q, Li N, et al. Angiotensin II receptor blocker as a novel therapy in acute lung injury induced by avian influenza A H5N1 virus infection in mouse. Sci China Life Sci 2015;58:208-11. [Crossref] [PubMed]
- Li L, Chong HC, Ng SY, et al. Angiopoietin-like 4 increases pulmonary tissue leakiness and damage during influenza pneumonia. Cell Rep 2015;10:654-63. [Crossref] [PubMed]
- Mehrbod P, Hair-Bejo M, Ibrahim TAT, et al. Simvastatin modulates cellular components in influenza A virus-infected cells. Int J Mol Med 2014;34:61-73. [PubMed]
- Radigan KA, Urich D, Misharin AV, et al. The effect of rosuvastatin in a murine model of influenza A infection. PLoS One 2012;7:e35788. [Crossref] [PubMed]
- Herold S, Steinmueller M, von Wulffen W, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med 2008;205:3065-77. [Crossref] [PubMed]
- Sato K, Nuki T, Gomita K, et al. Statins reduce endothelial cell apoptosis via inhibition of TRAIL expression on activated CD4 T cells in acute coronary syndrome. Atherosclerosis 2010;213:33-9. [Crossref] [PubMed]
- Fedson DS. Pandemic influenza: a potential role for statins in treatment and prophylaxis. Clin Infect Dis 2006;43:199-205. [Crossref] [PubMed]
- Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res 2013;99:417-35. [Crossref] [PubMed]
- Fedson DS. How will physicians confront the next influenza pandemic? Clin Infect Dis 2014;58:233-7. [Crossref] [PubMed]
- Morens DM, Taubenberger JK, Fauci AS. H7N9 avian influenza A virus and the perpetual challenge of potential human pandemicity. MBio 2013;4:e00445-13. [Crossref] [PubMed]
- Palache A, Oriol-Mathieu V, Fino M, et al. Seasonal influenza vaccine dose distribution in 195 countries (2004-2013): Little progress in estimated global vaccination coverage. Vaccine 2015;33:5598-605. [Crossref] [PubMed]
- WHO. Pandemic influenza risk management. Interim guidance. Available online: http://www.who.int/influenza/preparedness/pandemic/GIP_PandemicInfluenzaRiskManagementInterimGuidance_Jun2013.pdf, 2013.
- WHO. Influenza vaccine response during the start of a pandemic. Available online: who.int/iris/bitstream/10665/207751/1/WHO_OHE_PED_GIP_2016.1_eng.pdf?ua=1, 29 June-1 July 2015.apps.
- Joshi R, Venkatesan S, Myles P. A UK general practice population cohort study investigating the association between lipid lowering drugs and 20-day mortality following medically attended acute respiratory illness. Peer J 2016;4:e1902. [Crossref] [PubMed]
- Omer SB, Phadke VK, Bednarczyk RA, et al. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016;213:1216-23. [Crossref] [PubMed]
- Black S, Nicolay U, Del Giudice G, et al. Influence of statins on influenza vaccine responses in elderly individuals. J Infect Dis 2016;213:1224-8. [Crossref] [PubMed]
- McLean HQ, Chow BD, VanWormer JJ, et al. The effect of statin use on influenza vaccine effectiveness. J Infect Dis 2016;214:1150-8. [Crossref] [PubMed]
- Vandermeer ML, Thomas AR, Kamimoto L, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis 2012;205:13-9. [Crossref] [PubMed]
- Laidler MR, Thomas A, Baumbach J, et al. Statin treatment and mortality: propensity score-matched analyses of 2007–2008 and 2009–2010 laboratory-confirmed influenza hospitalizations. Open Forum Infect Dis 2015;2:ofv028. [Crossref] [PubMed]
- Bray M, Mahanty S. Ebola hemorrhagic fever and septic shock. J Infect Dis 2003;188:1613-17. [Crossref] [PubMed]
- Hill-Batorski L, Halfmann P, Neumann G, et al. The cytoprotective enzyme heme-oxygenase-1 suppresses Ebola virus replication. J Virol 2013;87:13795-802. [Crossref] [PubMed]
- Grosser N, Hemmerle A, Berndt G, et al. The antioxidant defense protein heme oxygenase 1 is a novel target for statins in endothelial cells. Free Radic Biol Med 2004;37:2064-71. [Crossref] [PubMed]
- Hacke M, Bjorkholm P, Hellwig A, et al. Inhibition of Ebola virus glycoprotein-mediated cytotoxicity by targeting its transmembrane domain and cholesterol. Nat Commun 2015;6:7688. [Crossref] [PubMed]
- Rasmussen AL, Okumura A, Ferris MT, et al. Host genetic diversity enables Ebola hemorrhagic pathogenesis and resistance. Science 2014;346:987-91. [Crossref] [PubMed]
- Escudero-Pérez B, Volchkova VA, Dolnik O, et al. Shed GP of Ebola virus triggers immune activation and increased vascular permeability. PLoS Pathog 2014;10:e1004509. [Crossref] [PubMed]
- Kreuels B, Wichmann D, Emmerich P, et al. A case of severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med 2014;371:2394-401. [Crossref] [PubMed]
- Uyeki TM, Mehta AK, Davey RT Jr, et al. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med 2016;374:636-46. [Crossref] [PubMed]
- Fedson DS. A practical treatment for patients with Ebola virus disease. J Infect Dis 2015;211:661-2. [Crossref] [PubMed]
- Enserink M. Debate erupts on ‘repurposed’ drugs for Ebola. Science 2014;345:718-9. [Crossref] [PubMed]
- Fedson DS, Jacobson JR, Rordam OM, et al. Treating the Host Response to Ebola Virus Disease with Generic Statins and Angiotensin Receptor Blockers. MBio 2015;6:e00716. [Crossref] [PubMed]
- Fedson DS, Rordam OM. Treating Ebola patients: a “bottom up” approach using generic statins and angiotensin receptor blockers. Int J Infect Dis 2015;36:80-4. [Crossref] [PubMed]
- Johansen LM, Brannan JM, Delos SE, et al. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med 2013;5:190ra79. [Crossref] [PubMed]
- Nelson EA, Barnes AB, Wiehle RD, et al. Clomiphene and its isomers block Ebola virus particle entry and infection with similar potency: potential therapeutic implications. Viruses 2016;8:206. [Crossref] [PubMed]
- Ray A, Ficek M. Immunomodulatory effects of anti-estrogenic drugs. Acta Pharm 2012;62:141-55. [Crossref] [PubMed]
- The Times – SL. Freetown, Sierra Leone. 1, 3, 5 and 8 February 2016. Available online: http://www.sierraleonetimes.com, accessed on April 22, 2016.
- World Health Organization. WHO consultation on potential Ebola therapies and vaccines. Meeting summary. Geneva. 4-5 September 2014. Available online: http://www.who.int/csr/resources/publications/ebola/ebola-therapies/en/, accessed 20 January 2016.
- Mendoza EJ, Qiu X, Kobinger GP. Progression of Ebola therapeutics during the 2014-2015 outbreak. Trends Mol Med 2016;22:164-73. [Crossref] [PubMed]
- Sissoko D, Laouenan C, Folkesson E, et al. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PLoS Med 2016;13:e1001967. [Crossref] [PubMed]
- Bai CQ, Mu JS, Kargbo D, et al. Clinical and Virological Characteristics of Ebola Virus Disease Patients Treated With Favipiravir (T-705)-Sierra Leone, 2014. Clin Infect Dis 2016;63:1288-1294. [Crossref] [PubMed]
- Dunning J, Sahr F, Rojek A, et al. Experimental treatment of Ebola virus disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med 2016;13:e1001997. [Crossref] [PubMed]
- Cohen J, Enserink M. As Ebola epidemic draws to a close, a thin scientific harvest. Science 2016;351:12-3. [Crossref] [PubMed]
- Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016;531:381-5. [Crossref] [PubMed]
- Howell KA, Qiu X, Brannan JM, et al. Antibody treatment of Ebola and Sudan virus infection via a uniquely exposed epitope within the glycoprotein receptor-binding site. Cell Rep 2016;15:1514-26. [Crossref] [PubMed]
- Geisbert TW, Strong JE, Feldmann H. Considerations in the use of nonhuman primate models of Ebola virus and Marburg virus infection. J Infect Dis 2015;212:S91-7. [Crossref] [PubMed]
- Fisher-Hoch SP, Mitchell SW, Sasso DR, et al. Physiological and immunologic disturbances associated with shock in a primate model of Lassa fever. J Infect Dis 1987;155:465-74. [Crossref] [PubMed]
- Ji XH, Sun KY, Feng YH, et al. Changes of inflammation-associated cytokine expressions during early phase of experimental endotoxic shock in macaques. World J Gastroenterol 2004;10:3026-33. [Crossref] [PubMed]
- Yin GQ, Qiu HB, Du KH, et al. Endotoxic shock model with fluid resuscitation in Macaca mulatta. Lab Anim 2005;39:269-79. [Crossref] [PubMed]
- Rotger M, Dalmau J, Rauch A, et al. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J Clin Invest 2011;121:2391-400. [Crossref] [PubMed]
- Mir KD, Mavigner M, Wang C, et al. Reduced simian immunodeficiency virus replication in macrophages of sooty mangabeys is associated with increased expression of host restriction factors. J Virol 2015;89:10136-44. [Crossref] [PubMed]
- Reperant LA, van de Bildt MW, van Amerongen G, et al. Marked endotheliotropism of highly pathogenic avian influenza virus H5N1 following intestinal inoculation in cats. J Virol 2012;86:1158-65. [Crossref] [PubMed]
- Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med 2014;20:214-23. [Crossref] [PubMed]
- Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012;487:477-81. [Crossref] [PubMed]
- Vuille-dit-Bille RN, Camargo SM, Emmenegger L, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids 2015;47:693-705. [Crossref] [PubMed]
- Wong TP, Ho KY, Ng EK, Debnam ES, Leung PS. Upregulation of ACE2-ANG-(1-7)-Mas axis in jejunal enterocytes of type 1 diabetic rats: implications for glucose transport. Am J Physiol Endocrinol Metab 2012;303:E669-81. [Crossref] [PubMed]
- Khajah MA, Fateel MM, Ananthalakshmi KV, et al. Anti-inflammatory action of angiotensin 1-7 in experimental colitis. PLoS One 2016;11:e0150861. [Crossref] [PubMed]
- Garg M, Burrell LM, Velkoska E, et al. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. J Renin Angiotensin Aldosterone Syst 2015;16:559-69. [Crossref] [PubMed]
- Wang W, Sun L, Xiao W, et al. Essential role of angiotensin receptors in the modulation of intestinal epithelial cell apoptosis. J Pediatr Gastroenterol Nutr 2013;57:562-9. [Crossref] [PubMed]
- de Araújo RF Jr, Reinaldo MP, Brito GA, et al. Olmesartan decreased levels of IL-1β and TNF-α, down-regulated MMP-2, MMP-9, COX-2, RANK/RANKL and up-regulated SOCs-1 in an intestinal mucositis model. PLoS One 2014;9:e114923. [Crossref] [PubMed]
- Arab HH, Al-Shorbagy MY, Abdallah DM, Nassar NN. Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS One 2014;9:e97193. [Crossref] [PubMed]
- Jahovic N, Gedik N, Ercan F, et al. Effects of statins on experimental colitis in normocholesterolemic rats. Scand J Gastroenterol 2006;41:954-62. [Crossref] [PubMed]
- Côté-Daigneault J, Mehandru S, Ungaro R, et al. Potential immunomodulatory effects of statins in inflammatory bowel disease. Inflamm Bowel Dis 2016;22:724-32. [Crossref] [PubMed]
- Lei A, Yang Q, Li X, et al. Atorvastatin promotes the expansion of myeloid-derived suppressor cells and attenuates colitis. Immunology 2016. [Epub ahead of print]. [Crossref] [PubMed]
- McElroy AK, Harmon JR, Flietstra TD, et al. Kinetic analysis of biomarkers in a cohort of US patients with Ebola virus disease. Clin Infect Dis 2016;63:460-7. [Crossref] [PubMed]
- Filewod NC, Lee WL. Is strengthening the endothelial barrier a therapeutic strategy for Ebola? Int J Infect Dis 2015;36:78-9. [Crossref] [PubMed]
- Hellman J. Addressing the complications of Ebola and other hemorrhagic fever infections: using insights from bacterial and fungal sepsis. PLoS Pathog 2015;11:e1005088. [Crossref] [PubMed]
- Leligdowicz A, Fischer WA II, Uyeki TM, et al. Ebola virus disease and critical illness. Crit Care 2016;20:217. [Crossref] [PubMed]
- Song MS, Baek YH, Pascua PN, et al. Growth and pathogenic potential of naturally selected reassortants after coinfection with pandemic H1N1 and highly pathogenic avian influenza H5N1 viruses. J Virol 2015;90:616-23. [Crossref] [PubMed]
- World Health Organization. WHO publishes list of top emerging diseases likely to cause major epidemics. Available online: http://www.who.int/medicines/ebola-treatment/WHO-list-of-top-emerging-diseases.en, accessed on 26 January 2016.
- Connolly-Andersen AM, Moll G, Andersson C, et al. Crimean-Congo hemorrhagic fever virus activates endothelial cells. J Virol 2011;85:7766-74. [Crossref] [PubMed]
- Sancakdar E, Guven AS, Uysal EB, et al. Important (sic) of angiopoietic system in evaluation of endothelial damage in children with Crimean-Congo hemorrhagic fever. Pediatr Infect Dis J 2015;34:e200-5. [Crossref] [PubMed]
- McLay L, Liang Y, Ly H. Comparative analysis of disease pathogenesis and molecular mechanisms of New World and Old World arenavirus infections. J Gen Virol 2014;95:1-15. [Crossref] [PubMed]
- Maisner A, Neufeld J, Weingartl H. Organ- and endotheliotropism of Nipah virus infections in vivo and in vitro. Thromb Haemost 2009;102:1014-23. [PubMed]
- Dalrymple NA, Mackow ER. Virus interactions with endothelial cell receptors: implications for viral pathogenesis. Curr Opin Virol 2014;7:134-40. [Crossref] [PubMed]
- Liu T, Warburton RR, Hill NS, et al. Anthrax lethal toxin-induced lung injury and treatment by activating MK2. J Appl Physiol (1985) 2015;119: 412-9. [PubMed]
- Delaney JW, Pinto R, Long J, et al. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care 2016;20:75. [Crossref] [PubMed]
- Whitehorn J, Nguyen CVV, Khanh LP, et al. Lovastatin for the treatment of adult patients with dengue: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2016;62:468-76. [PubMed]
- Shirey KA, Lai W, Scott AJ, et al. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 2013;497:498-502. [Crossref] [PubMed]
- Opal SM, Laterre PF, Francois B, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 2013;309:1154-62. [Crossref] [PubMed]
- Toner P, McAuley DF, Shyamsundar M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit Care 2015;19:374. [Crossref] [PubMed]
- Kor DJ, Carter RE, Park PK, et al. Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department. The LIPS-A randomized clinical trial. JAMA 2016;315:2406-14. [Crossref] [PubMed]
- Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014;2:395-404. [Crossref] [PubMed]
- Darwish I, Liles WC. Emerging therapeutic strategies to prevent infection-related microvascular endothelial activation and dysfunction. Virulence 2013;4:572-82. [Crossref] [PubMed]
- Lovegrove FE, Tangpukdee N, Opoka RO, et al. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 2009;4:e4912. [Crossref] [PubMed]
- Erdman LK, Dhabangi A, Musoke C, et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: a retrospective case-control study. PLoS One 2011;6:e17440. [Crossref] [PubMed]
- Kim H, Higgins S, Liles WC, Kain KC. Endothelial activation and dysregulation in malaria: a potential target for novel therapeutics. Curr Opin Hematol 2011;18:177-85. [Crossref] [PubMed]
- Hawkes M, Elphinstone RE, Conroy AL, Kain KC. Contrasting pediatric and adult cerebral malaria: the role of the endothelial barrier. Virulence 2013;4:543-55. [Crossref] [PubMed]
- Gomes LT, Alves-Junior ER, Rodrigues-Jesus C, et al. Angiopoietin-2 and angiopoietin-2/angiopoietin-1 ratio as indicators of potential severity of Plasmodium vivax malaria in patients with thrombocytopenia. PLoS One 2014;9:e109246. [Crossref] [PubMed]
- Hanson J, Lee SJ, Hossain MA, et al. Microvascular obstruction and endothelial activation are independently associated with the clinical manifestations of severe falciparum malaria in adults: an observational study. BMC Med 2015;13:122. [Crossref] [PubMed]
- Graham SM, Chen J, Chung DW, et al. Endothelial activation, haemostasis and thrombosis biomarkers in Ugandan children with severe malaria participating in a clinical trial. Malar J 2016;15:56. [Crossref] [PubMed]
- Kim H, Erdman LK, Lu Z, et al. Functional roles for C5a and C5aR but not C5L2 in the pathogenesis of human and experimental cerebral malaria. Infect Immun 2014;82:371-9. [Crossref] [PubMed]
- Souraud JB, Briolant S, Dormoi J, et al. Atorvastatin treatment is effective when used in combination with mefloquine in an experimental cerebral malaria murine model. Malar J 2012;11:13. [Crossref] [PubMed]
- Reis PA, Estato V, da Silva TI, et al. Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria. PLoS Pathog 2012;8:e1003099. [Crossref] [PubMed]
- Dormoi J, Briolant S, Pascual A, Desgrouas C, Travaillé C, Pradines B. Improvement of the efficacy of dihydroartemisinin with atorvastatin in an experimental cerebral malaria murine model. Malar J 2013;12:302. [Crossref] [PubMed]
- Wilson NO, Solomon W, Anderson L, et al. Pharmacologic inhibition of CXCL10 in combination with anti-malarial therapy eliminates mortality associated with murine model of cerebral malaria. PLoS One 2013;8:e60898. [Crossref] [PubMed]
- Canavese M, Crisanti A. Vascular endothelial growth factor (VEGF) and lovastatin suppress the inflammatory response to Plasmodium berghei infection and protect against experimental cerebral malaria. Pathog Glob Health 2015;109:266-74. [Crossref] [PubMed]
- Silva-Filho JL, Souza MC, Ferreira-Dasilva CT, et al. Angiotensin II is a new component involved in splenic T lymphocyte responses during Plasmodium berghei ANKA infection. PLoS One 2013;8:e62999. [Crossref] [PubMed]
- Higgins SJ, Purcell LA, Silver KL, et al. Dysregulation of angiopoietin-1 plays a mechanistic role in the pathogenesis of cerebral malaria. Sci Transl Med 2016;8:358ra128. [Crossref] [PubMed]
- Gallego-Delgado J, Basu-Roy U, Ty M, et al. Angiotensin receptors and β-catenin regulate brain endothelial integrity in malaria. J Clin Invest 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Hawkes MT, Conroy AL, Opoka RO, et al. Inhaled nitric oxide as adjunctive therapy for severe malaria: a randomized controlled trial. Malar J 2015;14:421. [Crossref] [PubMed]
- Moxon CA, Chisala NV, Wassmer SC, et al. Persistent endothelial activation and inflammation after Plasmodium falciparum infection in Malawian children. J Infect Dis 2014;209:610-5. [Crossref] [PubMed]
- Rosenke K, Adjemian J, Munster VJ, et al. Plasmodium parasitemia associated with increased survival in Ebola virus-infected patients. Clin Infect Dis 2016;63:1026-33. [Crossref] [PubMed]
- Gignoux E, Azman AS, de Smet M, et al. Effect of artesunate-amodiaquine on mortality related to Ebola virus disease. N Engl J Med 2016;374:23-32. [Crossref] [PubMed]
- Souza MC, Paixão FH, Ferraris FK, et al. Artesunate Exerts a Direct Effect on Endothelial Cell Activation and NF-κB Translocation in a Mechanism Independent of Plasmodium Killing. Malar Res Treat 2012;2012:679090.
- Fineberg HV. Pandemic preparedness and response--lessons from the H1N1 influenza of 2009. N Engl J Med 2014;370:1335-42. [Crossref] [PubMed]
- WHO Ebola Response Team. After Ebola in West Africa--unpredictable risks, preventable epidemics. N Engl J Med 2016;375:587-96. [Crossref] [PubMed]
- Checchi F, Waldman RJ, Roberts LF, et al. World Health Organization and emergency health: if not now, when? BMJ 2016;352:i469. [Crossref] [PubMed]
- Kamradt-Scott A. WHO’s to blame? The World Health Organization and the 2014 Ebola outbreak in West Africa. Third World Quarterly 2016;37:401-18. [Crossref]
- WHO. Report of the Ebola interim assessment panel. Geneva: World Health Organization. Available online: http://who.int/csr/resources/publications/ebola/report-by-panel.pdf, 2015.
- Moon S, Sridhar D, Pate MA, et al. Will Ebola change the game? Ten essential reforms before the next pandemic. The report of the Harvard-LSHTM Independent Panel on the Global Response to Ebola. Lancet 2015;386:2204-21. [Crossref] [PubMed]
- Sands P, Mundaca-Shah C, Dzau VJ. The neglected dimension of global security--a framework for countering infectious-disease crises. N Engl J Med 2016;374:1281-7. [Crossref] [PubMed]
- United Nations. Protecting humanity from future health crises. Report of the high-level panel on the global response to health crises. Available online: http://www.un.org/News/dh/infocus/HLP/2016-02-05_Final_Report_Global_Response_to_Health_Crises.pdf, 25 January 2016.
- Gostin LO, Tomori O, Wibulpolprasert S, et al. Toward a common secure future: four global commissions in the wake of Ebola. PLoS Med 2016;13:e1002042. [Crossref] [PubMed]
- International Rescue Committee. The Ebola Lessons Reader. What’s being said, what's missing and why it matters. Available online: ttp://www.rescue.org/resource-file/ebola-lessons-reader, March 2016.h
- Kieny MP, Rottingen JA, Farrar J, et al. The need for global R&D coordination for infectious diseases with epidemic potential. Lancet 2016;388:460-1. [Crossref] [PubMed]
- Balasegaram M, Bréchot C, Farrar J, et al. A global biomedical R&D fund and mechanism for innovations of public health importance. PLoS Med 2015;12:e1001831. [Crossref] [PubMed]
- Dunning JW, Merson L, Rohde GG, et al. Open source clinical science for emerging infections. Lancet Infect Dis 2014;14:8-9. [Crossref] [PubMed]
- Horby PW, Endzt H, Muyembe-Tamfum JJ, et al. Ebola: Europe-Africa research collaborations. Lancet Infect Dis 2015;15:1258-9. [Crossref] [PubMed]
- Caplan AL, Plunkett C, Levin B. Selecting the right tool for the job. Am J Bioeth 2015;15:4-10. [Crossref] [PubMed]
- Cooper BS, Boni MF, Pan-ngum W, et al. Evaluating clinical trial designs for investigational treatments of Ebola virus disease. PLoS Med 2015;12:e1001815. [Crossref] [PubMed]
- Berry SM, Petzold EA, Dull P, et al. A response adaptive randomization platform trial for efficient evaluation of Ebola virus treatments: A model for pandemic response. Clin Trials 2016;13:22-30. [Crossref] [PubMed]
- Dodd LE, Proschan MA, Neuhaus J, et al. Design of a randomized controlled trial for Ebola virus disease medical countermeasures: PREVAIL II, the Ebola MCM study. J Infect Dis 2016;213:1906-13. [Crossref] [PubMed]
- Grossman CM. The first use of penicillin in the United States. Ann Intern Med 2008;149:135-6. [Crossref] [PubMed]
- Glasziou P, Chalmers I, Rawlins M, et al. When are randomised trials unnecessary? Picking signal from noise. BMJ 2007;334:349-51. [Crossref] [PubMed]
- Vandenbroucke JP. Case reports in an evidence-based world. J R Soc Med 1999;92:159-63. [PubMed]
- Wunsch H, Linde-Zwirble WT, Angus DC. Methods to adjust for bias and confounding in critical care health services research involving observational data. J Crit Care 2006;21:1-7. [Crossref] [PubMed]
- Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 2011;26:546-50. [Crossref] [PubMed]
- Boyko EJ. Observational research--opportunities and limitations. J Diabetes Complications 2013;27:642-8. [Crossref] [PubMed]
- Vetter P, Kaiser L, Schibler M, et al. Sequelae of Ebola virus disease; the emergency within the emergency. Lancet Infect Dis 2016;16:e82-91. [Crossref] [PubMed]
- Borkar DS, Tham VM, Shen E, et al. Association between statin use and uveitis: results from the Pacific Ocular Inflammation study. Am J Ophthalmol 2015;159:707-13. [Crossref] [PubMed]
- Miyazaki A, Kitaichi N, Ohgami K, et al. Anti-inflammatory effect of angiotensin type 1 receptor antagonist on endotoxin-induced uveitis in rats. Graefes Arch Clin Exp Ophthalmol 2008;246:747-57. [Crossref] [PubMed]
- Lv S, Liu Y, Zou Z, et al. The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol 2015;33:69-76. [PubMed]
- Ishihara Y, Itoh K, Ishida A, et al. Selective estrogen-receptor modulators suppress microglial activation and neuronal cell death via an estrogen receptor-dependent pathway. J Steroid Biochem Mol Biol 2015;145:85-93. [Crossref] [PubMed]
- Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Law GL, Tisoncik-Go J, Korth MJ, et al. Drug repurposing: a better approach for infectious disease drug discovery? Curr Opin Immunol 2013;25:588-92. [Crossref] [PubMed]
- Ludwig S, Zell R, Schwemmle M, et al. Influenza, a One Health paradigm--novel therapeutic strategies to fight a zoonotic pathogen with pandemic potential. Int J Med Microbiol 2014;304:894-901. [Crossref] [PubMed]
- Wang Y, Zhang MX, Meng X, et al. Atorvastatin suppresses LPS-induced rapid upregulation of Toll-like receptor 4 and its signaling pathway in endothelial cells. Am J Physiol Heart Circ Physiol 2011;300:H1743-52. [Crossref] [PubMed]
- Gao P, Wu X, Shui H, et al. Fluvastatin inhibits angiotensin II-induced nuclear factor kappa B activation in renal tubular epithelial cells through the p38 MAPK pathway. Mol Biol Rep 2012;39:4719-25. [Crossref] [PubMed]
- Shao Q, Shen LH, Hu LH, et al. Atorvastatin suppresses inflammatory response induced by oxLDL through inhibition of ERK phosphorylation, IκBα degradation, and COX-2 expression in murine macrophages. J Cell Biochem 2012;113:611-8. [Crossref] [PubMed]
- Ozbek E, Cekmen M, Ilbey YO, et al. Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF-kappaB pathways. Ren Fail 2009;31:382-92. [Crossref] [PubMed]
- Shatanawi A, Romero MJ, Iddings JA, et al. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway. Am J Physiol Cell Physiol 2011;300:C1181-92. [Crossref] [PubMed]
- Tian XY, Wong WT, Xu A, et al. Rosuvastatin improves endothelial function in db/db mice: role of angiotensin II type 1 receptors and oxidative stress. Br J Pharmacol 2011;164:598-606. [Crossref] [PubMed]
- Zhang GX, Kimura S, Murao K, et al. Effects of angiotensin type I receptor blockade on the cardiac Raf/MEK/ERK cascade activated via adrenergic receptors. J Pharmacol Sci 2010;113:224-33. [Crossref] [PubMed]
- Zhang H, Ma G, Yao Y, et al. Olmesartan attenuates the impairment of endothelial cells induced by oxidized low density lipoprotein through downregulating expression of LOX-1. Int J Mol Sci 2012;13:1512-23. [Crossref] [PubMed]
- Lakshmanan AP, Thandavarayan RA, Watanabe K, et al. Modulation of AT-1R/MAPK cascade by an olmesartan treatment attenuates diabetic nephropathy in streptozotocin-induced diabetic mice. Mol Cell Endocrinol 2012;348:104-11. [Crossref] [PubMed]
- Shen L, Mo H, Cai L, et al. Losartan prevents sepsis-induced acute lung injury and decreases activation of nuclear factor kappaB and mitogen-activated protein kinases. Shock 2009;31:500-6. [Crossref] [PubMed]
- Davenport EE, Burnham KL, Radhakrishnan J, et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med 2016;4:259-71. [Crossref] [PubMed]
- Prescott HC, Calfee CS, Thompson BT, et al. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med 2016;194:147-55. [Crossref] [PubMed]
- Li D, Chen T, Hu Y, et al. An Ebola virus-like particle-based reporter system enables evaluation of antiviral drugs in vivo under non-biosafety Level 4 conditions. J Virol 2016;90:8720-8. [Crossref] [PubMed]
- Cheng F, Murray JL, Zhao J, et al. Systems biology-based investigation of cellular antiviral drug targets identified by gene-trap insertional mutagenesis. PLoS Comput Biol 2016;12:e1005074. [Crossref] [PubMed]
- Joyner MJ, Paneth N, Ioannidis JPA. What happens when underperforming big ideas in research become entrenched? JAMA 2016;316:1355-6. [Crossref] [PubMed]
- Joyner MJ, Pedersen BK. Ten questions about systems biology. J Physiol 2011;589:1017-30. [Crossref] [PubMed]
- Joyner MJ, Prendergast FG. Chasing Mendel: five questions for personalized medicine. J Physiol 2014;592:2381-8. [Crossref] [PubMed]
- Joyner MJ. Has Neo-Darwinism failed clinical medicine: Does systems biology have to? Prog Biophys Mol Biol 2015;117:107-12. [Crossref] [PubMed]
- Glasziou P, Altman DG, Bossuyt P, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014;383:267-76. [Crossref] [PubMed]
- Ioannidis JP. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank Q 2016;94:485-514. [Crossref] [PubMed]
- Baddeley M. Herding, social influences and behavioural bias in scientific research. EMBO Rep 2015;16:902-5. [Crossref] [PubMed]
- Boudreau KJ, Guinan EC, Lakhani KR, et al. Looking Across and Looking Beyond the Knowledge Frontier: Intellectual Distance, Novelty, and Resource Allocation in Science. Manage Sci 2016;62:2765-2783. [Crossref] [PubMed]
- Vandenbroucke JP, Broadbent A, Pearce N. Causality and causal inference in epidemiology: the need for a pluralistic approach. Int J Epidemiol 2016. [Epub ahead of print]. [PubMed]
- Howick J, Glasziou P, Aronson JK. Can understanding mechanisms solve the problem of extrapolating from study to target populations (the problem of 'external validity')? J R Soc Med 2013;106:81-6. [Crossref] [PubMed]
- Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence 2013;4:507-16. [Crossref] [PubMed]
- Rodrigues SF, Granger DN. Blood cells and endothelial barrier function. Tissue Barriers 2015;3:e978720. [Crossref] [PubMed]
- Norata GD, Caligiuri G, Chavakis T, et al. The cellular and molecular basis of translational immunometabolism. Immunity 2015;43:421-34. [Crossref] [PubMed]
- O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol 2016;16:553-65. [Crossref] [PubMed]
- Li H, Horke S, Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci 2013;34:313-9. [Crossref] [PubMed]
- Ishizaka N, Hongo M, Matsuzaki G, et al. Effects of the AT(1) receptor blocker losartan and the calcium channel blocker benidipine on the accumulation of lipids in the kidney of a rat model of metabolic syndrome. Hypertens Res 2010;33:263-8. [Crossref] [PubMed]
- Zhang O, Zhang J. Atorvastatin promotes human monocyte differentiation toward alternative M2 macrophages through p38 mitogen-activated protein kinase-dependent peroxisome proliferator-activated receptor γ activation. Int Immunopharmacol 2015;26:58-64. [Crossref] [PubMed]
- Aki K, Shimizu A, Masuda Y, et al. ANG II receptor blockade enhances anti-inflammatory macrophages in anti-glomerular basement membrane glomerulonephritis. Am J Physiol Renal Physiol 2010;298:F870-82. [Crossref] [PubMed]
- Forero-Peña DA, Gutierrez FR. Statins as modulators of regulatory T-cell biology. Mediators Inflamm 2013;2013:167086.
- Sun W, Lee TS, Zhu M, et al. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation 2006;114:2655-62. [Crossref] [PubMed]
- Henao-Restrepo AM, Preziosi MP, Wood D, et al. On a path to accelerate access to Ebola vaccines: The WHO's research and development efforts during the 2014-2016 Ebola epidemic in West Africa. Curr Opin Virol 2016;17:138-44. [Crossref] [PubMed]
- Currie J, Grenfell B, Farrar J. Infectious diseases. Beyond Ebola. Science 2016;351:815-6. [Crossref] [PubMed]
- Fedson DS, Dunnill P. Commentary: From scarcity to abundance: pandemic vaccines and other agents for “have not” countries. J Public Health Policy 2007;28:322-40. [Crossref] [PubMed]
- Fedson DS. Meeting the challenge of influenza pandemic preparedness in developing countries. Emerg Infect Dis 2009;15:365-71. [Crossref] [PubMed]
- Li M, Huang Y, Du X, et al. Impact of prior use of four preventive medications on outcomes in patients hospitalized for acute coronary syndrome--results from CPACS-2 Study. PLoS One 2016;11:e0163068. [Crossref] [PubMed]



