Epidermal growth factor as a prognostic biomarker in chronic kidney diseases
Chronic kidney disease (CKD), a major global public health problem (1), affects approximately 10% of the adult population worldwide (2). CKD increases the risk of all-cause mortality, cardiovascular disease, and the progression to end stage kidney diseases (1). The 2002 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines define CKD as chronic kidney damage, especially exhibiting albuminuria, or reduced glomerular filtration rate (GFR), and assign CKD stages based on the level of GFR. Meta-analysis of the clinical studies demonstrated that estimated GFR (eGFR) <60 mL/min/1.73 m2 and urine albumin-creatinine ratio ≥10 mg/g were significantly associated with an increased risk of all-cause and cardiovascular mortality independently of each other and traditional risk factors (2).
In order to improve the outcome of CKD patients, it is important to identify high risk patients at early stage of CKD, and to provide an effective therapy. Until today, however, we don’t have enough biomarker; for example, neutrophil gelatinase-associated lipocalin and liver-type fatty acid-binding protein showed even lower correlation factors with eGFR than urinary albumin (3). However, the association between albuminuria and CKD has low sensitivity and specificity (4). Urine sample is attractive as a rich source of biomarker for human kidney diseases, because urine collection is not invasive, and may reflect the kidney injury. Studies, directing the differences in urine samples between healthy subjects and CKD patients, showed the risk factors associating with the progression of kidney diseases (5,6). The diagnostic/prognostic analysis between urinary peptides and eGFR using large cohort, however, may lack pathological point. On the other hand, the investigation using biopsy specimen can obtain the histological information, but is invasive. And the population of the cohort may be small.
Ju et al. in Sci Transl Med (7) demonstrated that urine epidermal growth factor (EGF) is a noninvasive prognostic biomarker for CKD progression using a renal biopsy transcriptome-driven approach from the European Renal cDNA Bank (ERCB). They examined the ability of the candidate gene transcript to predict patients’ eGFR at time of biopsy. Among six genes [nicotinamide N-methyltransferase (NNMT), EGF, thymosin β 10 (TMSB10), tissue inhibitor of metalloproteinases 1 (TIMP1), tubulin α 1a (TUBA1A), and annexin A1 (ANXA1)] showing the best predictive ability, they selected EGF, because (I) EGF mRNA expression was correlated with eGFR in other two validation cohorts; (II) EGF expression was kidney-specific, and restricted to the thick ascending limb of Henle and distal tubules (8); and (III) EGF enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function after injury (9,10). Of interest, they demonstrated that urine EGF protein-to-creatinine ratio (uEGF/Cr) correlated significantly with the intrarenal EGF mRNA expression in 34 C-PROBE and 85 Nephrotic Syndrome Study Network (NEPTUNE) patients (7). As altered urinary EGF excretion is concordant with altered intrarenal EGF expression, uEGF/Cr may in part reflect transcript of EGF from the injured tubules per se, and be illustrating the intrinsic renal pathological changes. Among the candidate gene transcript, EGF expression was downregulated according to the decreased GFR. In case of upregulated transcripts, urine protein may be affected by glomeruli-filtrated protein, and may not add significant prognostic value to albuminuria. It was reported that serum EGF level was quite low compared with urine EGF levels, and no correlation between serum and urine concentration existed (11). Correlation between uEGF/Cr and eGFR was shown in independent three cohorts composed of diabetic nephropathy, focal segmental glomerulonephritis, minimal change nephrotic syndrome, membranous glomerulonephritis, and IgA nephropathy. In addition, uEGF/Cr showed only a weak negative correlation with urinary albumin-to-creatinine ratio. These observations suggest that uEGF/Cr could reflect the pathological changes common in a variety of kidney diseases, and represent a different pathophysiologic mechanism than that detected by albuminuria. In fact, uEGF/Cr was correlated with histological scores of interstitial fibrosis and tissue atrophy. Furthermore, uEGF/Cr predicted the CKD progression assessed by eGFR slope.
EGF activates cells via the EGF receptor (EGFR), which is involved in the various biological processes including proliferation, metabolism, differentiation, and survival (12). EGF is a potent mitogen to proximal tubular cells in primary culture (13). Many reports showed that EGFR signaling was also involved in the development and progression of renal disease. Proximal tubular cells (13) as well as cells from more distal tubular cells express EGFR. EGF expression was observed in the thick ascending limb of Henle and distal tubules (8), EGF activates tubular cell proliferation in autocrine/paracrine manner. Decreased renal expression and urinary excretion of EGF has been observed in various human kidney diseases, including diabetic nephropathy (14), IgA nephropathy (15), acute kidney injury (16), and lupus nephritis (17). Recently, Betz et al. (14) demonstrated that uEGF decreased early in the development of the disease in the Cyp1a1mRen2 model, in which streptozotocin-induced hyperglycemia develops in parallel with renin-dependent hypertension. Interestingly, EGF expression was also reduced at the renal transcriptional level in this rat model. These observations confirmed the results of Ju et al. (7). The clinical utility of a biomarker for CKD depends on its ability to predict the risk of the development of end stage kidney disease. An important strength of the study by Ju et al. (7) is to have examined the renal EGF expression and uEGF excretion in several large cohort, and showed the predictive value of uEGF in a variety of CKDs.
The activation of EGFR has been reported to result in favorable or harmful outcomes, depending on the individual situation (Figure 1). Acute kidney injury model showed that EGFR activation promotes renal tubular cell proliferation. Activation of EGFR by exogenous EGF, can promote recovery from acute kidney injury (9). However, recent studies demonstrated that EGFR activation leads to the development and progression of renal diseases (15). In the development of diabetic nephropathy, hypertensive nephropathy, or polycystic kidney disease, EGFR activation may be involved. Numerous stimuli can activate the EGFR, beyond EGF itself, including high glucose and angiotensin II as well as EGF ligands like TNF-α. Dominant negative EGFR overexpression mice exhibited less interstitial fibrosis than wild type mice after subtotal renal ablation or following angiotensin II infusion (18). It was also reported that EGF-EGFR axis regulates sodium transport and development of hypertension (19). In this point of view, decreased EGF may be beneficial by inhibiting the activation of EGFR. Future studies may elucidate the mechanism of EGF-EGFR axis in the development of CKD.
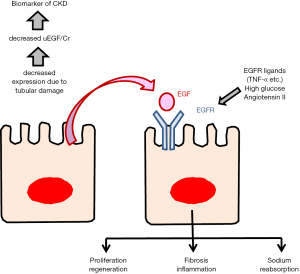
It was reported that tubulointerstitial score rather than glomerular injury correlated with the decline of the renal function (20). Thus, it is reasonable that tubular proteins such as EGF, reflecting tubular cell damage, may be better predictive biomarkers than albuminuria. It makes sense that uEGF/Cr correlated with the EGF expression in renal biopsy specimen and eGFR at the time of renal biopsy, and that predicted the eGFR decline in the study by Ju et al. (7). Furthermore, these observations were validated in other cohorts consisting of a variety of kidney diseases. uEGF seems useful to predict the CKD progression. However, it remains unclear whether uEGF can improve the current clinical setting in managing the CKD patients. It is also uncertain whether decreased EGF expression only reflects the damaged tubules or decreased EGF affects the development of tubulointerstitial injury. Future studies may dissolve the questions whether treatment to increase uEGF/Cr or supplementation of EGF may improve the prognosis of the CKD patients.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Commentary commissioned by Section Editor Zhaohui Huang, MD (Wuxi Oncology Institute, Affiliated Hospital of Jiangnan University, Wuxi, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007;72:247-59. [Crossref] [PubMed]
- Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073-81. [Crossref] [PubMed]
- Chou KM, Lee CC, Chen CH, et al. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. PLoS One 2013;8:e54863. [Crossref] [PubMed]
- Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes 2000;49:1399-408. [Crossref] [PubMed]
- Klein J, Ramirez-Torres A, Ericsson A, et al. Urinary peptidomics provides a noninvasive humanized readout of diabetic nephropathy in mice. Kidney Int 2016;90:1045-1055. [Crossref] [PubMed]
- Schanstra JP, Zürbig P, Alkhalaf A, et al. Diagnosis and Prediction of CKD Progression by Assessment of Urinary Peptides. J Am Soc Nephrol 2015;26:1999-2010. [Crossref] [PubMed]
- Ju W, Nair V, Smith S, et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 2015;7:316ra193. [Crossref] [PubMed]
- Salido EC, Lakshmanan J, Fisher DA, et al. Expression of epidermal growth factor in the rat kidney. An immunocytochemical and in situ hybridization study. Histochemistry 1991;96:65-72. [Crossref] [PubMed]
- Humes HD, Cieslinski DA, Coimbra TM, et al. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest 1989;84:1757-61. [Crossref] [PubMed]
- Safirstein R, Zelent AZ, Price PM. Reduced renal prepro-epidermal growth factor mRNA and decreased EGF excretion in ARF. Kidney Int 1989;36:810-5. [Crossref] [PubMed]
- Joh T, Itoh M, Katsumi K, et al. Physiological concentrations of human epidermal growth factor in biological fluids: use of a sensitive enzyme immunoassay. Clin Chim Acta 1986;158:81-90. [Crossref] [PubMed]
- Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem 1990;265:7709-12. [PubMed]
- Norman J, Badie-Dezfooly B, Nord EP, et al. EGF-induced mitogenesis in proximal tubular cells: potentiation by angiotensin II. Am J Physiol 1987;253:F299-309. [PubMed]
- Betz BB, Jenks SJ, Cronshaw AD, et al. Urinary peptidomics in a rodent model of diabetic nephropathy highlights epidermal growth factor as a biomarker for renal deterioration in patients with type 2 diabetes. Kidney Int 2016;89:1125-35. [Crossref] [PubMed]
- Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int 2013;83:804-10. [Crossref] [PubMed]
- Kwon O, Ahn K, Zhang B, et al. Simultaneous monitoring of multiple urinary cytokines may predict renal and patient outcome in ischemic AKI. Ren Fail 2010;32:699-708. [Crossref] [PubMed]
- Reich HN, Landolt-Marticorena C, Boutros PC, et al. Molecular markers of injury in kidney biopsy specimens of patients with lupus nephritis. J Mol Diagn 2011;13:143-51. [Crossref] [PubMed]
- Lautrette A, Li S, Alili R, et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 2005;11:867-74. [Crossref] [PubMed]
- Staruschenko A, Palygin O, Ilatovskaya DV, et al. Epidermal growth factors in the kidney and relationship to hypertension. Am J Physiol Renal Physiol 2013;305:F12-20. [Crossref] [PubMed]
- Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 1992;20:1-17. [Crossref] [PubMed]


