Circulating fibrocytes serve as a marker for clinical diagnosis
Cardiovascular disease continues to be a major health problem in the United States and the leading cause of death (1). According to the American Heart Association, 86.5 million or more than one in three Americans have one or more cardiovascular diseases. Of the 86.5 million, 8.2 million suffer from angina pectoris. The American Heart Association predicts 43.9% of Americans will be afflicted with cardiovascular disease by 2030 (1). Despite marked progress towards the understanding of cardiovascular pathophysiology and rapid improvement in medical and surgical options, the number is still increasing. Therefore, discovery of an early diagnostic tool is important to prevent disease. Fibrocytes are progenitor cells which primarily function in response to inflammation. A recent study by Keeley et al. aimed to identify markers in unstable angina that may be used to predict future adverse outcomes (2). They demonstrated that the total number of fibrocytes strongly correlates with recurrent angina and unfavorable clinical events independent of risk factors. There is also evidence of expansion of circulating fibrocytes which express an activated phenotype and myofibroblast differentiation (2). These findings further support the authors’ reasoning that fibrocytes have a role in vascular remodeling and their usefulness as markers. However, fibrocytes play an extensive part in immunity and utility as markers for specific pathologies may be difficult.
Identification of fibrocytes
Circulating fibrocytes are reported for first time in 1994 and are characterized as a distinct population of spindle-shaped cells with the phenotype of CD45+, collagen+, and CD34+ that are present within the blood (3). Fibrosis contributes to the pathology of a variety of diseases (4), particularly inflammatory. Due to the importance of the role of fibrocytes in tissue remodeling, much work is done to investigate the significance of fibrocyte participation in different diseases and to establish markers to detect, determine prognosis, and prevent adverse clinical outcomes. In general, mature fibrocytes have the markers CD34, CD43, CD45, LSP-1, and major histocompatibility complex (MHC) class II, which contributes to their hematopoietic nature and collagen type I and III, which explains their stromal behavior (5). Their ability to migrate to sites of injury is because they contain the markers CCR2, CCR7, and CXCR4. When fibrocytes home to sites of injury and differentiate, they change the expression of their markers. For instance, some may lose CD34 and CD45 and some may express markers to mimic the cells they specialize (5). As a result, their dynamic expression presents an obstacle to track their activity (6). Additionally, fibrocytes are derived from monocytes, thus have characteristics of hematopoietic cells and macrophages along with features of fibroblasts. Therefore, finding specific markers of fibrocytes is especially arduous. Despite the challenge, one study has found that they can be distinguished from other cells because of the unique combination of CD45RO, 25F9, and S100A8/A9 expression (7). However, the discovery of more specific markers is yet to be determined.
Function of fibrocytes
Circulating fibrocytes are progenitor cells that originate from bone marrow, which circulate within the bloodstream and principally function to generate components of the extracellular matrix such as vimentin, collagen type I, and collagen type II (8,9). They are derived from monocyte precursors and have characteristics of both macrophages and fibroblasts (10). Under inflammatory conditions, these cells participate in tissue healing and repair. In response to injury, fibrocytes migrate to the inflammatory site via induction by stromal cell-derived factor 1 alpha (SDF-1α) (6). Once there, fibrocytes enhance leukocyte trafficking via increased expression of leukocyte adhesion molecules and recruitment of inflammatory cells through production of interleukin 6 (IL-6), IL-8, CC-chemokine ligand 3 (CCL3), and CCL4 (10). Repair function is initiated in fibrocytes by IL-10 and the presence of apoptotic cells (10). Additionally, neovascularization is promoted by a pro-angiogenic factor, vascular endothelial growth factor (VEGF), released by fibrocytes to aid in the repair process (6). Thus, these cells regulate immune responses via secretion of cytokines and growth factors and stimulate repair through activation of fibroblasts (11). Similar to macrophages, fibrocytes are also involved in antigen presentation to CD8+ T cells and lipid metabolism (10).
Due to the mesenchymal properties of fibrocytes, they are capable of forming myofibroblasts, osteoblasts, and adipocytes (7). The differentiation and activity of fibrocytes are primarily determined by signals in the microenvironment, which activate intracellular pathways (7). For example, a study found that transforming growth factor beta 1 (TGF-β1) increases the expression of type I collagen, alpha-smooth muscle actin (αSMA), and tissue inhibitor of metalloproteinase-1 (TIMP-1) in fibrocytes which indicates transformation into myofibroblasts (6). Whereas, another study found that fibrocytes are under the influence of TGF-β3, which leads to upregulation of col2A1 and aggrecan and differentiation into chondrocytes (12). Fibrocytes also have the ability to differentiate into osteoblasts when stimulated by Runx2/core binding factor alpha 1 (Cbfα1) and osterix, transcription factors essential for producing bone matrix (12). Exposure of fibrocytes to peroxisome proliferator-activated receptor gamma (PPAR-γ) stimulates transformation into adipocytes (12). Direct regulation of fibrocyte differentiation has been related to CD4+ lymphocytes, which supports differentiation (7). The schematic diagram depicts the origin and differentiation of circulating fibrocytes (Figure 1).
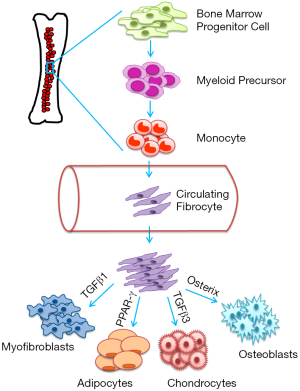
Clinical implications
Studies have shown that circulating fibrocytes have a role in many cardiac diseases, particularly those involving fibrosis such as coronary heart disease, hypertensive heart disease, and cardiac ischemia (13-15). As such, markers of fibrocytes can provide valuable information regarding the extent of disease and course of treatment. In the study by Keely et al., fibrocytes were examined under the condition of unstable angina with a focus on their differentiation to myofibroblasts. The combinations of the markers CD45, αSMA, and collagen 1 were used to identify the fibrocytes and the levels of TGF-β1 to determine the breadth of expansion to myofibroblasts (2). Since both fibrocytes and myofibroblasts have general functions in healing and repair, their markers may be found in several diseases. For example, a study done in neonates with bronchopulmonary dysplasia, increased fibrocytes with αSMA has been demonstrated when compared to healthy subjects (9). However, the authors recognized that patients with known existing fibrotic diseases or other conditions may trigger fibrocyte activity and excluded them from the study.
Levels of circulating fibrocytes are relatively stable under normal conditions and rises with inflammation and hypoxic situations (13). This phenomenon can be taken advantage of in order to indirectly measure the extent of disease. Furthermore, the study by Keeley et al. has shown that the number of total fibrocytes correlates strongly with adverse outcomes in patients with unstable angina (2). However, the relationship is not causative and perhaps the total number of fibrocytes measures the extent of cardiac fibrosis and thus more likely to lead to unfavorable clinical events with higher numbers. Another investigation showed a positive correlation of CXCR4/procollagen-1 and CXCR4/αSMA fibrocytes with SDF-1/CXCL12+ expression by infarcted cells in coronary heart disease (16).
Fibrocyte markers can be applied in a clinical setting to help determine prognosis and clinical course. The ability to foresee adverse outcomes as a product of unstable angina can lead to prevention by more aggressive treatments to those at risk (2). In addition, circulating fibrocytes may be used to improve wound healing and perhaps prevent pathological fibrosis (8). More importantly, fibrocytes help determine the magnitude of fibrotic reactions (17) and may be a potential target to inhibit excessive fibrosis as seen in many inflammatory diseases. For example, class I histone deacetylase (HDAC) inhibitors regulate differentiation of fibrocytes and leads to a reduction of both fibrocytes in the heart and circulating fibrocytes when cardiac fibrosis is induced with angiotensin II (18). Moreover, fibrocytes may have the potential in regenerative medicine due to their ability to form other cell types. For instance, the capacity to form chondrocytes and osteoblasts can be used in repair, especially in damage to the articular cartilage (12). In the last few years, there have been many studies aimed to understand the importance of circulating fibrocytes in various diseases, including cardiovascular diseases. However, the currently available data suggest that circulating fibroblast might be a novel and promising therapeutic target and a marker for treatment response and prognostic evaluation.
Conclusions
Circulating fibrocytes have important biologic roles, but can also contribute to diseases related to fibrosis. Therefore, understanding these functions under normal conditions can help to prevent aberrant fibrotic processes and identification of more specific cell-surface markers may be used to predict the clinical course. The clinical importance of fibrocytes is not limited to cardiac disease, but can be virtually applied to all fibrotic diseases and may extend into regenerative medicine.
Acknowledgements
Funding: This work was supported, in part, by American Heart Association Grant-in-Aid 16GRNT30950010 and National Institutes of Health COBRE grant P20GM104936 (to J Rajasingh).
Footnote
Provenance: This is a Guest Commentary commissioned by Section Editor Zhijun Han, MD (Department of Laboratory Medicine, Wuxi Second Hospital, Nanjing Medical University, Wuxi, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016;133:447-54. [Crossref] [PubMed]
- Keeley EC, Schutt RC, Marinescu MA, et al. Circulating fibrocytes as predictors of adverse events in unstable angina. Transl Res 2016;172:73-83.e1. [Crossref] [PubMed]
- Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994;1:71-81. [PubMed]
- Alhamad EH, Shakoor Z, Al-Kassimi FA, et al. Rapid detection of circulating fibrocytes by flowcytometry in idiopathic pulmonary fibrosis. Ann Thorac Med 2015;10:279-83. [PubMed]
- Pilling D, Fan T, Huang D, et al. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One 2009;4:e7475. [Crossref] [PubMed]
- Suga H, Rennert RC, Rodrigues M, et al. Tracking the elusive fibrocyte: identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells 2014;32:1347-60. [Crossref] [PubMed]
- Smith TJ. Potential role for bone marrow-derived fibrocytes in the orbital fibroblast heterogeneity associated with thyroid-associated ophthalmopathy. Clin Exp Immunol 2010;162:24-31. [Crossref] [PubMed]
- Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep 2006;8:145-50. [Crossref] [PubMed]
- Li C, Li X, Deng C, et al. Circulating Fibrocytes Are Increased in Neonates with Bronchopulmonary Dysplasia. PLoS One 2016;11:e0157181. [Crossref] [PubMed]
- Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol 2011;11:427-35. [Crossref] [PubMed]
- Pilling D, Gomer RH. Differentiation of circulating monocytes into fibroblast-like cells. Methods Mol Biol 2012;904:191-206. [PubMed]
- Choi YH, Burdick MD, Strieter RM. Human circulating fibrocytes have the capacity to differentiate osteoblasts and chondrocytes. Int J Biochem Cell Biol 2010;42:662-71. [Crossref] [PubMed]
- Lin RJ, Su ZZ, Liang SM, et al. Role of Circulating Fibrocytes in Cardiac Fibrosis. Chin Med J (Engl) 2016;129:326-31. [Crossref] [PubMed]
- Chu PY, Mariani J, Finch S, et al. Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol 2010;176:1735-42. [Crossref] [PubMed]
- van Amerongen MJ, Bou-Gharios G, Popa E, et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol 2008;214:377-86. [Crossref] [PubMed]
- Lei PP, Qu YQ, Shuai Q, et al. Fibrocytes are associated with the fibrosis of coronary heart disease. Pathol Res Pract 2013;209:36-43. [Crossref] [PubMed]
- Baker DW, Tsai YT, Weng H, et al. Alternative strategies to manipulate fibrocyte involvement in the fibrotic tissue response: pharmacokinetic inhibition and the feasibility of directed-adipogenic differentiation. Acta Biomater 2014;10:3108-16. [Crossref] [PubMed]
- Williams SM, Golden-Mason L, Ferguson BS, et al. Class I HDACs regulate angiotensin II-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J Mol Cell Cardiol 2014;67:112-25. [Crossref] [PubMed]


