Immunotherapy in small-cell lung cancer: at what point are we?
Immunotherapy is a new frontier for the management of cancers with practice-changing trials already reported for unresectable or metastatic malignant melanoma, advanced non-small-cell lung cancer (NSCLC), and advanced renal-cell carcinoma (1). Considering these recent exciting developments, immunotherapy is being investigated in small-cell lung cancer (SCLC), too. Unfortunately, in the last decades, despite several treatment attempts and new strategic approaches investigated, SCLC outcomes have not improved. In fact, even if an objective response rate (ORR) with first-line chemotherapy was reached by most of the patients, they relapse within a year of treatment. At relapse, the response to first-line chemotherapy and its duration are the main factors in predicting the efficacy of salvage chemotherapy. In fact, based on these two factors patients who respond to initial chemotherapy and relapse more than 60–90 days after the end of chemotherapy are defined as ‘sensitive relapse’ patients, while those whose tumor is stable or progresses during the initial chemotherapy or who have a recurrence within 60–90 days after the end of chemotherapy are considered ‘refractory relapse’ patients (2). A meta-analysis collecting the data from six trials involving intravenous topotecan-based second-line chemotherapy showed that treatment-free interval (TFI) <60 days is the cut-off to consider SCLC patients as refractory to second-line chemotherapy and with poorer overall survival (OS) (3). Overall, recurrent SCLC patients have a very poor prognosis. In this general discouraging context, immunotherapy might be particularly suitable especially for SCLC patients, who are generally characterized by a worst prognosis (2).
Cytotoxic T-lymphocyte antigen-4 (CTLA-4) receptor is a checkpoint molecule controlling the activation and proliferation of T-cells (4). Two anti-CTLA-4 monoclonal antibodies are currently being investigated in SCLC: ipilimumab, a fully human immunoglobulin G, and tremelimumab, a fully human immunoglobulin G2. Another important immune checkpoint pathway interaction is the one between the programmed death-1 (PD-1) receptor, expressed on activated T-cells, and its ligands, the programmed death-1 ligand (PD-L1) and the PD-L2, produced by stromal and tumor cells (4). Among the PD-1 inhibitors, two monoclonal antibodies are under investigation for SCLC: nivolumab, a fully human IgG4, and pembrolizumab, a humanized IgG4.
In order to reach a higher anti-tumor activity, and based on promising preclinical data (5), the combination of a PD-1 and of a CTLA-4 inhibitor was investigated. In fact, the combination of nivolumab and ipilimumab is already approved in the USA and the European Union for treatment of advanced melanoma (6). These results led to design the phase I/II CheckMate-032 study to investigate the activity and safety of the single-agent nivolumab or the combination of nivolumab with ipilimumab in several advanced cancers including SCLC.
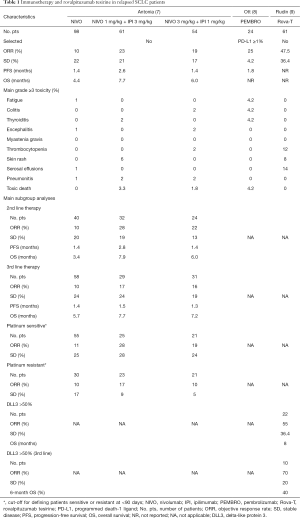
Antonia et al. published the results of patients affected by SCLC and enrolled in the CheckMate-032 trial (7). In this study, both limited- or extensive-stage SCLC patients who progressed after at least one previous platinum-containing regimen could be enrolled. Patients were either assigned to single-agent nivolumab, at the dose of 3 mg/kg, every 2 weeks, given until disease progression or unacceptable toxicity, or assessed in a dose-escalating safety phase of nivolumab plus ipilimumab beginning at nivolumab 1 mg/kg plus ipilimumab 1 mg/kg. Depending on tolerability, patients were then assigned to nivolumab 1 mg/kg plus ipilimumab 3 mg/kg or nivolumab 3 mg/kg plus ipilimumab 1 mg/kg. The combination, independently from the doses, was recycled every 3 weeks for four cycles, followed by nivolumab 3 mg/kg every 2 weeks, given until disease progression or unacceptable toxicity. The primary endpoint was ORR by investigator assessment. In the ChekMate-032 trial, out of a total of 216 enrolled SCLC patients, 98 received nivolumab 3 mg/kg, three were treated with nivolumab 1 mg/kg plus ipilimumab 1 mg/kg, 61 received nivolumab 1 mg/kg plus ipilimumab 3 mg/kg, and 54 nivolumab 3 mg/kg plus ipilimumab 1 mg/kg. Most enrolled patients received ≥2 prior regimens. In the single-agent nivolumab arm, the ORR was 10%, with a stable disease (SD) reached in 22% of patients. The ORR was reached in one (33%) of the three patients enrolled in the lower doses of combination with SD in the other two patients (67%). In the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg group, the ORR was 23% with a SD of 21%, while in the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg group the ORR was 19% and the SD 17%. Median progression-free survival (PFS) was 1.4 months [95% confidence interval (CI): 1.4–1.9] in the single-agent nivolumab cohort, 2.6 months (95% CI: 1.4–4.1) in the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg cohort, and 1.4 months (95% CI: 1.3–2.2) in the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg cohort. Median OS was 4.4 months (95% CI: 3.0–9.3), 7.7 months (95% CI: 3.6–18.0), and 6.0 months (95% CI: 3.6–11.0) with the 1-year OS of 33%, 43%, and 35%, respectively. The combination of nivolumab and ipilimumab resulted more toxic. Grade 3–4 adverse events occurred in 13% of patients in the nivolumab 3 mg/kg cohort (mainly fatigue), 30% in the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg cohort, and 19% in the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg cohort (mainly diarrhea, increased lipase, vomiting and rash). No grade 3 or 4 treatment-related adverse event was reported in the three patients in the nivolumab 1 mg/kg plus ipilimumab 1 mg/kg cohort. Treatment was discontinued due to treatment-related adverse events in 6% of patients in the nivolumab 3 mg/kg group, 11% in the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg group, and 7% in the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg group. Two toxic deaths were reported in the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg cohort (myasthenia gravis and renal failure), and one in the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg group (pneumonitis). PD-L1 expression was assessable in 148 (69%) of 216 patient samples, of which 27% was from fresh biopsies and 74% from archived specimens. A total of 25 (17%) patients had PD-L1 expression ≥1%, and seven (5%) ≥5%. In a pre-planned exploratory analysis, ORRs occurred irrespective of PD-L1 expression. Moreover, outcomes were observed regardless of the number of previous lines of therapy, and considering only patients who received platinum-based first-line treatment, ORRs were achieved in both platinum-sensitive and platinum-resistant disease patients using a TFI <90 as cut-off (7) (Table 1). These results are particularly interesting in terms of activity and safety, with adverse events generally manageable, also considering the heavily pretreated and unselected SCLC population. However, some considerations should be made.
Full table
The main limitation of this study was the absence of a randomization to assign treatments. Thus, it was not designed for formal comparisons across cohorts. The KEYNOTE-028 phase Ib study enrolled 24 PD-L1-positive (≥1% at immunohistochemistry using the 22C3 antibody at a central laboratory) SCLC patients to assess the safety, tolerability, and ORR of pembrolizumab given at 10 mg/kg every 2 weeks for a maximum of 24 months. The preliminary results showed a grade 3–5 toxicity reported in 8.3% of patients including one toxic death (colitis). The ORR was 29.2% with a median PFS of 1.8 months (8) (Table 1). These further data seem to underline that probably firstly single-agent immunotherapy should be studied better to define the real role of immunotherapy in SCLC patients, and then go through combinations of immunotherapeutics which, as above reported, are already being investigated. Furthermore, monotherapy should have advantages respect to combination therapy, due to its convenience of administration and cost-effectiveness.
Another important issue is to define the role of PD-L1 expression in SCLC patients. The activity of nivolumab and its combination with ipilimumab seem not to be affected by PD-L1 expression, while pembrolizumab was investigated in patients with PD-L1 expression ≥1%. The prevalence of PD-L1 expression in SCLC reported in the KEYNOTE-028 study was 28.6% (42 out of 147 evaluable samples) and it is lower than that showed by NSCLC. However, these results cannot draw any robust conclusion because coming from small sample sizes of patients and from phase I trials.
The only two drugs registered for the treatment of second-line therapy of SCLC patients are topotecan, which is the standard-of-care for platinum-sensitive patients worldwide, and amrubicin, which is a valid alternative to topotecan but is registered for administration in this setting only in Japan. Looking at the SCLC outcomes reported by these drugs in the same setting in which immunotherapy was investigated, both as monotherapy or combination, the results are quite better with chemotherapy (2). In fact, immunotherapy seems to work better in terms of OS than PFS as showed also by some pivotal previous randomized trials such as those with nivolumab in advanced non-squamous NSCLC and renal-cell carcinoma (10,11). However, these considerations come from indirect comparisons and should be looked at only as hypotheses to be eventually confirmed in randomized trials.
A new targeted approach is very promising for the treatment of relapsed SCLC patients. Delta-like protein 3 (DLL3), a dipeptide linker, is expressed in about 80% of SCLC. This potential target seems to be higher expressed respect to PD-L1 expression. Rovalpituzumab tesirine is a first-in-class antibody-drug conjugate comprised of a humanized monoclonal antibody against DLL3, and a pyrrolobenzodiazepine dimer toxin. In a phase I study, 74 relapsed SCLC patients received rovalpituzumab tesirine at dose levels ranging from 0.05 to 0.8 mg/kg at either every 3 or 6 weeks. Among the 61 evaluable patients treated at doses of 0.2–0.4 mg/kg, the ORR was 25%. Twenty-two patients had ≥50% of cells expressing DLL3 by immunohistochemistry from archive tissue specimens showing an ORR of 55% with a median OS of 8 months. In the ten patients treated in third-line setting, where no approved therapy currently exists, the ORR was 70%. The most common grade ≥3 adverse events were serosal effusions in 14% of patients, thrombocytopenia in 12%, and skin reactions in 8% of cases (9) (Table 1). If confirmed in further ongoing trials, this result would challenge the role of immunotherapy in SCLC.
Toxic effects were mild and manageable using specifically safety suggested algorithms (12). However, the frequency of immune encephalitis and myasthenia gravis seems to be higher in patients with SCLC compared with other malignant diseases (1). Hypotheses for this finding could be due to the increased trend of paraneoplastic neurological syndromes associated with SCLC and to the potential role of prophylactic cranial irradiation. However, as per other cancers treated with immunotherapy, it is essential to closely monitor SCLC patients for immune-related toxicities and act with prompt implementation of adequate guidelines for their management (12).
Immunotherapy should be a new approach also for the management of SCLC patients. To understand if it might be “practice-changing” in this setting, it is important to plan trials comparing immunotherapy with standard-of-care therapies like those performed in other solid cancers (10,11). New agents, such as rovalpituzumab tesirine, should also be considered for challenging immunotherapy. It is also mandatory to select SCLC patients based on prognostic factors which, in second-line therapy, are the response to first-line chemotherapy and its duration. In this context, immunotherapy might be particularly suitable especially for “refractory relapse” SCLC patients who are characterized by the worst prognosis. To date, answering to the title question, we are just at the early beginning, and all these issues need further investigation in well-designed prospective larger trials.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by Section Editor Jianrong Zhang, MD (Department of Thoracic Surgery, First Affiliated Hospital of Guangzhou Medical University, Guangzhou Institute of Respiratory Disease, Guangzhou, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Li Y, Li F, Jiang F, et al. A Mini-Review for Cancer Immunotherapy: Molecular Understanding of PD-1/PD-L1 Pathway & Translational Blockade of Immune Checkpoints. Int J Mol Sci 2016.17. [PubMed]
- Rossi A, Sacco PC, Sgambato A, et al. Optimal drugs for second-line treatment of patients with small-cell lung cancer. Expert Opin Pharmacother 2016;17:969-76. [Crossref] [PubMed]
- Ardizzoni A, Tiseo M, Boni L. Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: a pooled analysis of topotecan second-line trials. Eur J Cancer 2014;50:2211-8. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275-80. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Ott PA, Elez E, Hiret S, et al. Pembrolizumab for ED SCLC: Efficacy and relationship with PD-L1 expression (NCT02054806). J Thorac Oncol 2015;10:S193.
- Rudin CM, Pietanza MC, Bauer TM, et al. Safety and efficacy of single-agent rovalpituzumab tesirine (SC16LD6.5), a delta-like protein 3 (DLL3)-targeted antibody-drug conjugate (ADC) in recurrent or refractory small cell lung cancer (SCLC). J Clin Oncol 2016;34:abstr LBA8505.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375-91. [PubMed]


